Horned Parakeet Eunymphicus cornutus Scientific name definitions
- VU Vulnerable
- Names (18)
- Monotypic
Text last updated November 14, 2014
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | cotorra banyuda |
| Czech | kakariki chocholatý |
| English | Horned Parakeet |
| English (United States) | Horned Parakeet |
| French | Perruche cornue |
| French (France) | Perruche cornue |
| German | Hornsittich |
| Japanese | ヘイワインコ |
| Norwegian | topparakitt |
| Polish | modrolotka czubata |
| Russian | Рогатый попугай |
| Serbian | Rogati eunimfikus papagaj |
| Slovak | kakariki uškatý |
| Spanish | Perico Cornudo |
| Spanish (Spain) | Perico cornudo |
| Swedish | hornparakit |
| Turkish | Sarı Başlı Sorguçlu Papağan |
| Ukrainian | Какарікі рогатий |
Eunymphicus cornutus (Gmelin, 1788)
Definitions
- EUNYMPHICUS
- cornuta / cornutus
The Key to Scientific Names
Legend Overview
Field Identification
32 cm; male 167 g, female 125 g (1). Generally green , more yellowish on underparts, with rump, ear-coverts and nape greenish yellow; forehead to mid-crown red with several (usually two), long, red-tipped black crest-feathers (60–70 mm); front of face around eye to chin smudgily black; outer webs of primaries violet blue; tail distally shaded violet, lateral feathers violet tipped whitish. Bare parts: bill blue-grey tipped black, iris orange, and legs and feet black. Sexes alike, but female smaller in most biometrics. Immature has greyer and less extensive facial markings, pale green ear-coverts, olive-green hindneck; bill horn-coloured, iris brown. Differs from sympatric <em>Cyanoramphus novaezelandiae saisseti</em> by head pattern, bluer, longer and more rounded tail, and larger, stouter structure, and from <em>Trichoglossus haematodus deplanchii</em> by lack of dark purple head and red breast, slower flight and different habits. Formerly conspecific E. uvaeensis, confined to island of Ouvea, lacks yellow tinging on head, with more crest feathers, all green, and red more restricted on crown.
Systematics History
Subspecies
Distribution
New Caledonia.
Habitat
Forests up to 30 m tall), notably Agathis australis and Araucaria-dominated, as well as savannas, low-stature forest and scrub in maquis and high mountains, but avoids coconut plantations and coastal areas. Commonest in humid valleys, especially on limestone soils, and recorded to 1500 m (2).
Movement
Sedentary, but it may migrate seasonally to foraging grounds during austral winter (Jun–Sep). Birds able to cross scrub between forest blocks, and species is not believed to be fragmented into distinct subpopulations.
Diet and Foraging
Berries of vines, seeds of various trees and shrubs, notably “penubre tree”, Agathis lanceolata, Lantana camara and Carica papaya (3). Typically observed in pairs or small flocks of up to 6–10 birds, feeding high in canopy (2), but sometimes observed in larger flocks feeding in savanna areas.
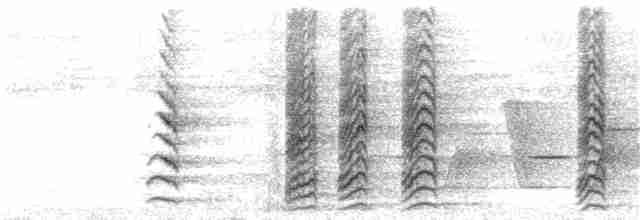
Sounds and Vocal Behavior
Noisy , giving repeated, nasal, raucous “khoo” or “kho-koot” calls, rather similar to those of New Caledonian Crow (Corvus moneduloides), but lower-pitched and more trumpeting, and more varied than those of sympatric Cyanoramphus novaezelandiae saisseti; also gives softer chattering notes and range of shrieks (in alarm) and chuckling calls (when perched or feeding) (2). Compared to E. uvaeensis, calls are slower in tempo (especially food-begging call) and higher-pitched (all call types), with the mate (contact) call being most different (1).
Breeding
Sept–Mar. Nest in hole in dead or living tree, species including Metrosideros demonstrans; sometimes on ground, including under rocks and in fallen tree-trunks (4). Two pairs sometimes share the same nest (5). Eggs 2–4, although usually only two young are reared, which reach maximum weight of 180 g (5); in captivity, incubation lasts 21–22 days (by female alone), nestling period 5–6 weeks. Breeding success: at 17 wild nests, mean clutch size 3·4 eggs, with mean number hatching 2·8 and mean number of fledglings 1·9 (5).
Conservation Status
VULNERABLE. CITES I. A BirdLife “restricted-range” species. Has declined substantially since the 1880s owing to habitat loss and cagebird trade and may now number c. 8000–9000 individuals, equivalent to 5300–6000 mature individuals. Range much reduced on Mt Panié (now restricted to NW part). Numbers and trends poorly known; prior to 2003–2006 just two independent population estimates of 1000–3000 birds and 720 pairs, respectively. A recent study using distance sampling density data, records, and ecological niche modelling indicates that species has wider distribution and is commoner than previously believed. During 2003–2006 surveys, species was recorded from Ignambi massif in N of New Caledonia to various massifs of Grand Sud in S, being recorded on 57% of massifs in N and 42% of those in S. Absent from Île des Pins. Locally common in central part of the “chaîne” (Mé Maoya Massif, Moindou-Farino area, Poindimié-Ponérihouen area), while numbers have been stable in Rivière Bleue over last 20 years. Any current decline might be due to habitat degradation, through logging and by introduced Rusa deer (Rusa timorensis), while black rats (Rattus rattus) occasionally prey on species’ nest, although this is unconfirmed. Particularly wet (La Niña) years reduce breeding success. Little documented trapping or trade (4) and, although there are captive birds on Grande Terre and birds are locally sought for trade, this seems to be marginal and since the species breeds in remote areas and its nests are hard to find, poaching is unlikely to be major threat. Occasional illegal hunting. The introduction of Psittacine Beak and Feather Disease could be threat, but to date tests for the disease have proved negative in this species (6).

- Year-round
- Migration
- Breeding
- Non-Breeding