Cape Robin-Chat Cossypha caffra Scientific name definitions
Text last updated October 1, 2018
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Gewone Janfrederik |
| Catalan | còssifa del Cap |
| Dutch | Kaapse Lawaaimaker |
| English | Cape Robin-Chat |
| English (Kenya) | Cape Robin Chat |
| English (United States) | Cape Robin-Chat |
| French | Cossyphe du Cap |
| French (France) | Cossyphe du Cap |
| German | Kaprötel |
| Icelandic | Sunnuskotta |
| Japanese | ツグミヒタキ |
| Norwegian | gråflanketrosteskvett |
| Polish | złotokosik ogrodowy |
| Russian | Капский рыжехвост |
| Serbian | Sivogrudi crvendać |
| Slovak | akalat záhradný |
| Spanish | Cosifa Cafre |
| Spanish (Spain) | Cosifa cafre |
| Swedish | kapsnårskvätta |
| Turkish | Kap Kızılca Bülbülü |
| Ukrainian | Золотокіс садовий |
Cossypha caffra (Linnaeus, 1771)
Definitions
- COSSYPHA
- cafer / caffer / caffra
The Key to Scientific Names
Legend Overview
Field Identification
16–17 cm; 25–34 g. Nominate race is olive-grey from crown to lower back, darker wings (with white post-carpal edge), chestnut rump and tail, latter with grey-brown central feathers; broad white supercilium, blackish mask; white narrow moustachial line bordering orange-white chin with dark whisker mark, shading to orange-buff breast, grey breast side and flanks , whitish belly and orange-buff vent and undertail-coverts; bill and legs brownish to blackish. Sexes similar. Juvenile is spotted buff above, scaled dusky below. Race namaquensis is like nominate but larger, with stronger supercilium; <em>iolaemus</em> is darker above and below than nominate; kivuensis similar to previous but more richly coloured below.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Proposed races vespera (E Zimbabwe), ardens (Transvaal) and drakensbergi (NE South Africa) synonymized with nominate. Four subspecies recognized.Subspecies
Cossypha caffra iolaema Scientific name definitions
Distribution
Cossypha caffra iolaema Reichenow, 1900
Definitions
- COSSYPHA
- cafer / caffer / caffra
- iolaema / iolaemus
- Iolaema
The Key to Scientific Names
Legend Overview
Cossypha caffra kivuensis Scientific name definitions
Distribution
Cossypha caffra kivuensis Schouteden, 1937
Definitions
- COSSYPHA
- cafer / caffer / caffra
- kivuense / kivuensis
The Key to Scientific Names
Legend Overview
Cossypha caffra namaquensis Scientific name definitions
Distribution
Cossypha caffra namaquensis Sclater, 1911
Definitions
- COSSYPHA
- cafer / caffer / caffra
- namaqua / namaquensis / namaquus
The Key to Scientific Names
Legend Overview
Cossypha caffra caffra Scientific name definitions
Distribution
Cossypha caffra caffra (Linnaeus, 1771)
Definitions
- COSSYPHA
- cafer / caffer / caffra
The Key to Scientific Names
Legend Overview
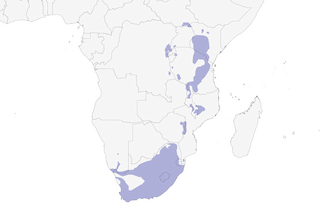
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Movement
Diet and Foraging
Wide variety of animal and vegetable food recorded, with opportunist use of local resource, e.g. caterpillars often taken when abundant in canopy, and termites often dominant in diet in acacia woodland; lizards and small frogs also recorded. In South Africa, of 17 stomachs and 87 faecal samples (mainly from KwaZulu-Natal), 88% held ants, 67% beetles (Coccinellidae, Curculionidae, Melolonthidae, Scarabaeidae, Staphylinidae, Tenebrionidae), 65% fruit, 46% moths and caterpillars, 35% termites, 17% parasitic wasps, 15% bugs (Naucoridae, Tingidae), 12% spiders, 10% orthopterans, 9% flies (Asilidae, Tabanidae), and 4% centipedes and pseudoscorpions; stomachs of 23 birds from throughout year (from Free State) held, by number, 37% seeds, 30% berries, 18% ants, 10% beetles, 2% plant parts, 1% unidentified larvae, 1% flies and 1% lepidopterans and bugs. Fruits consumed in Western Cape include Asparagus, Celtis, Cestrum, Ficus, Halleria, Hedychium, Ilex, Kiggelaria, Maytenus, Morus, Olea, Physalis, Psidium, Rhus, Rubus and Solanum, with seeds (including attached oil-rich funicles) of exotic Acacia cyclops. Forages mainly on ground under shrubs or in open, often in sight of mate, usually disturbing leaf litter by hopping through, rather than by using bill; perches low on branch or rock and sallies to ground and in air after prey; also gleans from foliage, twigs and trunks, and ascends to canopy for caterpillars. Follows army-ant swarms. On SW Cape coast, forages for crustacea and kelp-fly larvae in intertidal zone. Visits birdtables, taking foods such as cheese and bonemeal; raids domestic pets’ food-bowls, and recorded as entering house to peck butter.
Sounds and Vocal Behavior
Song , by both sexes (female normally shorter muted versions), a series of melodious whistled phrases, repetitive, halting and fairly high, most starting with soft, downslurred whistle, “tiiu-chiio chiio-tu-tiio tiio-tiio-tu-wiiuu”; delivery faster in N races than in S ones; mimicry variable in extent individually, non-existent to extensive, with up to 20 bird species copied. Main call a highly distinctive “wurdedur” or “garg-ga-garg” or “turr-da-da”, sometimes simply “garg”, and somewhat faster with a few more syllables N of R Zambezi, used in alarm, contact and at roost; in anxiety a soft descending “piiiiuuu”, and in distress a quiet ticking.
Breeding
Conservation Status
- Year-round
- Migration
- Breeding
- Non-Breeding





























