Mao Gymnomyza samoensis Scientific name definitions
- EN Endangered
- Names (18)
- Monotypic
Text last updated December 29, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | menjamel mao |
| Dutch | Samoahoningeter |
| English | Mao |
| English (United States) | Mao |
| French | Méliphage mao |
| French (France) | Méliphage mao |
| German | Maohonigfresser |
| Japanese | ハシグロオオミツスイ |
| Norwegian | maohonningeter |
| Polish | atolowiec czarny |
| Russian | Самоанский мао |
| Serbian | Samoanski džinovski medojed |
| Slovak | medárik mao |
| Spanish | Mielero Mao |
| Spanish (Spain) | Mielero mao |
| Swedish | samoahonungsfågel |
| Turkish | Mao Balkuşu |
| Ukrainian | Мао самоанський |
Gymnomyza samoensis (Hombron & Jacquinot, 1841)
Definitions
- Gymnomyza
- samoensis
The Key to Scientific Names
Legend Overview
Field Identification
28–31 cm. Large dark honeyeater with long, gently decurved bill. Appears wholly dark at distance: head, neck and breast sooty black to olive-black, conspicuous olive streak from below eye across upper ear-coverts; largely dusky olive-brown or dark brown upperbody and underbody, varyingly washed olive-green, with rump and uppertail-coverts slightly paler than rest of upperparts, and undertail-coverts slightly paler and browner than rest of underbody; uppertail and upperwing dark olive-brown, olive-green outer edges of rectrices, greater coverts and remiges (olive-green wash on folded wing); iris brownish-grey to brown, but often blue in males (1); bill black; legs black to blackish-grey, soles yellow. Sexes alike in plumage, male larger than female. Juvenile is like adult, but head and underbody less strongly black.
Systematics History
Subspecies
Distribution
Savaii and Upolu, in Samoa.
Habitat
Native forest and forest edges, and cultivated land with remnants of rainforest, e.g. in steep valleys, in foothills and montane areas; greatest densities in craters at high altitude in least disturbed forest. Also seen in cinder cone, in heathland scrub, and on steep slopes along rivers. Reported as visiting coconut trees near coast (probably in periods of stress after cyclones), and apparently was more widespread in lowlands in the past. Recorded at 760 m and above 900 m, and said to be commoner on the very tops of peaks; found in remaining rainforest at up to 1500m. Recently found to be present in upland areas with disturbed forest next to cattle farms and plantations.
Movement
Sedentary, with some local movements.
Diet and Foraging
Nectar, arthropods (mainly insects) and small fruit. Forages usually in crowns of trees, or in subcanopy; seen foraging in coral trees (Erythrina), the blossoms of which contain nectar and many insects; said to forage also on moss-covered branches. Forages also in Albizia chinensis and Paraserianthes falcataria; searches intensively in rotten wood of dead branches of these large trees, digging into the soft wood and probing with bill, sometimes while hanging head downwards; observed to extract and swallow insect larvae. Inconspicuous, heard more often than seen. Usually in pairs, partners seeming to stay close together. Competes with many other species at flowers of Erythrina, where it is dominant, attacking all other species except Samoan Starling (Aplonis atrifusca); in presence of a group of latter species, performs song flights as an indirect sign of territoriality.
Sounds and Vocal Behavior
Considered noisy. Call described as a loud cat-like wailing “mee-aa-oo” or a series of low, hoarse, nasal mewing notes, at times rising in pitch, followed by clearer high-pitched phrases and developing into loud, upslurred yelps before subsiding again; given in bouts lasting for up to 1 minute or more (one call comprising at least 55 notes), and heard most often in morning and evening. Other calls include nasal, downslurred “yaaw”; loud, mechanical-sounding “chlip”; and short squeaky notes. Acoustic analysis, measuring the central frequency of the alarm calls, allow sex discrimination with 100% confidence (1).
Breeding
Both sexes, but more intensely males, exhibit territorial defence through song, chasing and occasional physical contact; only females appear to build nests; nests are oval cup-shaped, measure c. 14 cm × 8 cm, contain little lining and are constructed at the junction of branches from interlaced young branches stripped of leaves; nests are constructed in a wide range of native and exotic trees, at 8–20 m above the ground in forest with canopy heights of c. 30 m; clutch, 1 egg (n = 10); eggs off-white with irregular shaped brown spots on the blunt end; incubation c. 20 days; newly hatched nestlings are blind and naked with a yellow culmen and pale-yellow flange, and at 9–10 days old the first feather tracts erupt and the culmen darkens; nestling period at least 22–24 days; female cares for the young for 2–2·5 months post-fledging, and apparently do not re-nest after successfully fledging a chick, though pairs attempt to re-nest following breeding failure (2).
Conservation Status
ENDANGERED. Restrictedrange species: present in Samoan Islands EBA. Rare. Total population small (estimate of 1000–2500 individuals), fragmented and apparently declining, and small subpopulations may not be viable. Formerly occurred on Tutuila, in American Samoa, but now extinct; last collected in 1924, sight records in 1933, and last unconfirmed report in 1977; status on Tutuila, however, not certainly known, and may have occurred only as a visitor from Samoa. Thought to have been formerly widespread in forests in Samoa, from coasts to mountains, but range dramatically reduced and the species appears to have become rarer within its current range. Decline largely attributed to loss of forest habitat, and degradation of remaining habitat, with Samoan forests now open and patchy, and so supporting fewer birds and becoming more vulnerable to weed invasion. Apparently dependent on remaining patches of primary forest , but remaining areas of upland forest threatened by slash-and-burn cultivation, farmers using roads from logged lowland forests to gain access to formerly inaccessible land; forest quality further reduced by invasion of aggressive exotic trees, spread of which is aided by cyclones and by the planting. Cyclones a significant threat; during the two most powerful recent cyclones, in 1990 and in 1991, forest canopy cover was reduced from 100% to 27%, and populations of present species were reduced, e.g. disappeared from lowland forests in O Le Pupu-pu’e National Park (on Upolu) between 1982 and 1991 following these two cyclones. Fires in low-rainfall forests, hunting (despite national bans on hunting native birds and bats that have been in place for more than ten years) and introduced rats (Rattus) may also pose a threat to the species. Occurs in some proposed and existing protected areas, but these have suffered damage from cyclones and O Le Pupu-pu’e National Park is threatened by logging and cattle-farming. Local peoples often shot this species when they saw it near villages, as it was considered a bird of ill omen.
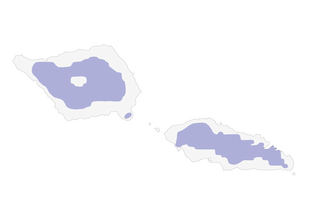
- Year-round
- Migration
- Breeding
- Non-Breeding




























