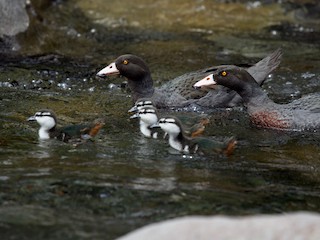Blue Duck Hymenolaimus malacorhynchos Scientific name definitions
Text last updated January 10, 2014
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Новозеландска синя патица |
| Catalan | ànec blau |
| Czech | kachna měkkozobá |
| Danish | Newzealandsk Strømand |
| Dutch | Blauwe Eend |
| English | Blue Duck |
| English (United States) | Blue Duck |
| Finnish | vuorisorsa |
| French | Canard bleu |
| French (France) | Canard bleu |
| German | Saumschnabelente |
| Icelandic | Dragönd |
| Japanese | アオヤマガモ |
| Norwegian | kaskadeand |
| Polish | krzywonos |
| Russian | Синяя утка |
| Serbian | Novozelandska patka iz brzaka |
| Slovak | vahia modrá |
| Spanish | Pato Azul |
| Spanish (Spain) | Pato azul |
| Swedish | blåand |
| Turkish | Mavi Ördek |
| Ukrainian | Качка новозеландська |
Hymenolaimus malacorhynchos (Gmelin, 1789)
Definitions
- HYMENOLAIMUS
- hymenolaimus
- malacorhynchos
The Key to Scientific Names
Legend Overview
Field Identification
53–54 cm; male 820–1077 g, female 680–870 g (1). Generally slate-blue with greenish gloss on head , neck and back, chestnut spots on breast , outer secondaries tipped white and narrow black margins to inner secondaries and tertials (1). Female averages smaller and has less breast spotting . Bare parts: bill pink-white with black flap at tip; legs and feet dark grey with black at joints; irides yellow (1). Juvenile lacks or virtually lacks breast spotting, and has no gloss on head and back; bill grey, irides dark brown (1). Subspecies hymenolaimus darker above.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Race hymenolaimus has been regarded as of uncertain status, and generally not accepted; recent phylogeographical study, however, revealed strong genetic differences between North I and South I populations (2). Two subspecies recognized.Subspecies
Hymenolaimus malacorhynchos hymenolaimus Scientific name definitions
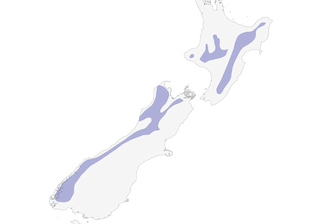
Distribution
central and eastern North Island, New Zealand
Hymenolaimus malacorhynchos hymenolaimus Mathews, 1937
Definitions
- HYMENOLAIMUS
- hymenolaimus
- malacorhynchos
The Key to Scientific Names
Legend Overview
Hymenolaimus malacorhynchos malacorhynchos Scientific name definitions
Distribution
western South Island, New Zealand
Hymenolaimus malacorhynchos malacorhynchos (Gmelin, 1789)
Definitions
- HYMENOLAIMUS
- hymenolaimus
- malacorhynchos
The Key to Scientific Names
Legend Overview
Distribution
Habitat
Mountain streams and small, narrow rivers of clear, fast-flowing waters in well-vegetated areas, usually on steep gradient in forested areas (1); rarely visits estuaries (3). Structured analysis of habitat, based on multi-area survey throughout New Zealand, found that sections of river supporting the species had significantly greater physical stability, channel gradient (>12 m km -1) and elevation (82–1050 m), narrower widths (8–60 m), coarser bed substrata (particularly more boulders) and more native riparian forest (> 50% of vegetation on average) than sections where it was absent (4). Also recorded at alpine tarns and lakes, occasionally farm pools, but does not breed in such areas (1) and perhaps most frequently observed in such areas during dispersal (5) (see Movements). For roosting, a recent study found that stable undercut banks are most commonly used (42%), followed by log jams along stream banks (25%), and large woody debris is a component of 50% of sites (6).
Movement
Mainly sedentary, adult birds occurring within breeding territory (c. 0·7–1 km linear section of river) throughout year and for life (1), unless displaced by adverse weather in winter; however, several transalpine movements of up to c. 25 km (mainly by juveniles) have been reported from the Southern Alps in the Arthur’s Pass region (5). Territories apparently highly fixed, even with changes of pairings due to death or displacement of one partner (1). Although (as noted above) juveniles may wander extensively within, and sometimes beyond, natal catchment during first year of life, they generally return to same area and attempt to establish territory nearby (1); tendency for juvenile males to move farther than young females, with a study in Fiordland National Park revealing that 87% of females remained in their natal catchment, moving a mean 3·84 ± 0·6 km, whereas only 40% of juvenile males remained in their natal catchment and a mean dispersal distance of 8·77 ± 2·05 km (7). Recent genetic study found that Tongariro R supports what is now a ‘sink’ population solely dependent upon immigration from several different source populations, rather than local production, although scale of movements necessary to support this is generally unknown (8).
Diet and Foraging
Aquatic invertebrates, mainly insect (chironoid and cased caddis fly) (1) larvae, but extensive range recorded (1); also some algae and berries of streamside plants (1) (e.g. Coprosma rugosa, C. depressa, Phyllocladus alpinus, Muehlenbeckia axillaris, Luzula crinita) (9), and also consumes some silt and periphyton (1). In one study, 37 taxa of aquatic invertebrates were identified within this duck’s diet, of which 45% were Chironornidae (samples ranged from 19–76%), 28% Trichoptera (range 11–49%) and 16% Ephemeroptera (range 2–42%), with the dominant chironomid Eukiefferiella sp., although Cricotopus spp. were also relatively abundant, and caddis flies were main Trichoptera in all samples; Plecoptera comprised 0–20% of invertebrates in faeces (10). Another study, along Manganuiateao R, in C North Island, also found that selectivity varied, but Trichoptera larvae of the Hydrobiosidae and genus Aoteapsyche (Hydropsychidae) ranked highly, and cased caddis larvae consistently ranked low, in the diet (11). Fruit-eating presumably most prevalent in autumn and analysis of droppings suggests may be, at least regionally, important dietary constituent at this season, perhaps especially in subalpine zone (9). Young take some foods as adults (1). Feeds by probing among, and scraping bill over (1), rocks, diving ; mainly by head-dipping , upending, dabbling and pecking (12) in shallow waters, especially at margins of rivers where current’s force dissipated by rocks (1); ingestion of stonefly and mayfly larvae apparently mainly associated with diving (11). Soft lateral flaps at bill’s tip function to increase surface area from which food is gathered, as well as to protect bill from abrasion (1); although it had been suggested that these flaps might serve as a tactile method of detecting prey, it appears that most food is detected visually and that the species has a wide binocular field of view (13). Foraging apparently occupies as little as 25% of daylight hours, mainly for 1–1·5 hours during first 1–2 hours after dawn, then another period of similar intense feeding after short break, with further bouts of prolonged feeding in late afternoon until dusk (1); nocturnal feeding also occurs, especially on rivers with high aggregate loads and generally low aquatic invertebrate densities (1).
Sounds and Vocal Behavior
Male gives high-pitched piercing, wheezing whistles in territorial defence or advertisement, with neck stretched forward and upper-neck feathers raised, and also utters a short “whi”or “whio” during interactions with intruders; female has long, low, rasping growl in response to disturbance or threat, and also gives low-pitched staccato rasping during other social interactions (1).
Breeding
Starts mostly mid Aug with majority of first nests attempted by late Oct, with repeat laying (following early loss of first clutches) perhaps extending to early Dec (1). Monogamous (14). In single pairs; nests in damp depression in ground, natural cavity, hollow log (1), crevice among rocks, in dense vegetation (e.g. fern clump) (1) or on cliff ledge, usually < 30 m from river edge (1); only a little down added to ground debris, sometimes with soft grass and twigs added (1); same site may be reused for up to seven years (1). Usually 5–6 eggs (4–9), white, laid at intervals of at least 24 hours, size 59–70·9 mm × 40·8–46·7 mm, mean mass 62·3 g (1); incubation c. 31–35 days (1), by female alone, with male remaining close by for at least 68% of each day, and female taking two periods off nest per day of 37–48 minutes each, but sometimes does not leave nest for two days prior to hatching (1); chicks have dark brown down above , white below ; fledging 70–82 days and young tended by both adults (1). Chief causes of nest failure are flooding and predation (1), especially by stoats (Mustela erminea) that can take eggs, ducklings and regularly adult females (15) (many subpopulations exhibit a male-biased sex ratio as a result) (16), as well as by brush-tailed possum (Trichosurus vulpecula) (17), and two other bird species, namely Weka (Gallirallus australis) and Kea (Nestor notabilis) (7). Predation pressure greatest in beech mast years, when population of mustelids increases in line with that of rodents, but temporarily remains high as numbers of rodents wane (7). Other causes of mortality included bacterial infections (17% in one study) and predation by New Zealand Falcon (Falco novaeseelandiae) (also 17%), with two birds being killed by avalanches (7). In one study, of 61 nests, 33 (54%) successfully hatched ducklings, with early nests being generally more successful (72%) and 92% of eggs hatched in successful nests; thereafter, in one study, only 12% of 33 broods lost and 60% of 55 ducklings that reach river actually fledge (1). Average of less than one young per pair reared in one study during five seasons, in another productivity was 1·3 fledglings/pair (n = 58) but varied dramatically between years (0–2·7 per pair) and pairs (0·25–2·3 young/year) over period of 5–9 years (1). Sexual maturity at one year, but 50% of yearlings do not attempt to breed, with rest doing so in following or third years (1). Mean annual survival between fledging and territory establishment 0·44 and of territorial adults 0·86, while mean lifespan after assuming territory is 6·75 years (1).
Conservation Status
ENDANGERED. Previously considered Near Threatened, as numbers very reduced and declining; formerly widespread, now rare or absent in majority of territory, dispersed in small, isolated populations in widely scattered locations in North I and South I. Population currently considered by BirdLife International to be 1200 mature individuals within overall range of 54,800 km2, but another recent estimate suggested the presence of 640 pairs on North Island and 700 pairs on South Island, with largest numbers in catchments of Bay of Plenty, C North Island, Northwest Nelson, West Coast and Fiordland, but an exhaustive survey of Arthur’s Pass National Park found only six birds in Feb 2011, down from c. 50 in early 1980s. Very sensitive to slightest modification in the torrents of clear water and thus threatened by hydroelectric schemes (e.g. the Tongariro Power Development scheme on North Island, around which local H. malacorhynchos population is in decline and characterized by low pair density and low annual productivity) (8) and mining activities. Previously, grazing and clearance of waterside vegetation decreased water quality and led to species’ disappearance from lowland rivers. Breeding success low; suffers high predation from introduced mammals, but in some areas (e.g. Fiordland National Park, where significant declines in Blue Ducks have been reported) (7) introduced stoats are being trapped, with positive results for nesting productivity (15), e.g. mean annual productivity in areas with no trapping compared to sites where stoats have been heavily trapped is 0·67 ± 0·17 nests, 0·22 ± 0·22 ducklings and no fledglings, versus 0·53 ± 0·07 nests, 1·17 ± 0·28 ducklings and 0·8 ± 0·24 fledglings, respectively (7). Elsewhere, a 2·8-fold population increase in four years with three fledged young per pair each year and 94% chick survival has been noted, whereas nest failures hit 91% and productivity was 0·64 fledged young/pair at unmanaged sites (16). These actions constitute part of two co-ordinated management programmes, Operation Ark and Whio Operation Nest Egg (WHIONE) (16). Operation Ark was initiated in 2003 to target species occurring in Nothofagus forest, and initially focused on establishing five viable populations of this duck (each of 50 pairs) with an extensive network of predator traps at each site (18). WHIONE has very successfully monitored breeding pairs, removed the eggs to hatch in captivity and then returned the young once they are less likely to be predated (16), which programme has operated alongside one of large-scale predator control. Furthermore, introduced trout may compete for food, but no studies carried out as yet, while introduced alga Didymo may reduce habitat quality (16). Major floods destroy nests and eggs, reduce aquatic invertebrate food resources, and can kill juvenile birds by displacing them from their parents; a population viability analysis has found that the additive effects of population losses due to predation and a rising frequency of floods, as expected from anthropogenic climate change, increases substantially extinction risk (19). Little gene flow between neighbouring catchments, with adjacent territorial pairs often closely related and sibling and cousin pairings are common (14). Translocation may prove a necessary conservation tool, but requires careful management and it has been suggested that genetic difference between North and South Island populations means that they should be treated as completely separate units for conservation purposes (20). Captive population established on North Island where c. 20 young reared annually, with (in 1987 and 1991) 12 birds released at Egmot National Park on Mt Taranaki and further releases annually since, with the result that breeding occurred for the first time in 2005–2006 when five pairs produced two young.
- Year-round
- Migration
- Breeding
- Non-Breeding