Collared Pratincole Glareola pratincola Scientific name definitions
Text last updated January 29, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Rooivlerksprinkaanvoël |
| Albanian | Dallëndyshe deti |
| Arabic | أبو اليسر مطوق |
| Armenian | Մարգագետնային ծիծառակտցար |
| Asturian | Perdiz de mar |
| Azerbaijani | Adi haçaquyruq cüllüt |
| Basque | Pratinkola |
| Bulgarian | Кафявокрил огърличник |
| Catalan | perdiu de mar europea |
| Chinese (SIM) | 领燕鸻 |
| Croatian | riđokrila pješčarka |
| Czech | ouhorlík stepní |
| Danish | Rødvinget Braksvale |
| Dutch | Vorkstaartplevier |
| English | Collared Pratincole |
| English (United States) | Collared Pratincole |
| Finnish | pääskykahlaaja |
| French | Glaréole à collier |
| French (France) | Glaréole à collier |
| Galician | Perdiz do mar común |
| German | Rotflügel-Brachschwalbe |
| Greek | Νεροχελίδονο |
| Hebrew | שדמית אדומת-כנף |
| Hungarian | Székicsér |
| Icelandic | Þernutrítill |
| Italian | Pernice di mare |
| Japanese | ニシツバメチドリ |
| Latvian | Brūnspārnu bezdelīgtārtiņš |
| Lithuanian | Pievinė kregždūnė |
| Malayalam | വാലൻ പെരുമീവൽക്കാട |
| Mongolian | Өнгөт хөгт |
| Norwegian | brakksvale |
| Persian | گلاریول بال سرخ |
| Polish | żwirowiec łąkowy |
| Portuguese (Angola) | Perdiz-do-mar-d'asa-vermelha |
| Portuguese (Brazil) | perdiz-do-mar |
| Portuguese (Portugal) | Perdiz-do-mar |
| Romanian | Ciovlică ruginie |
| Russian | Луговая тиркушка |
| Serbian | Zijavac |
| Slovak | prieložník stepný |
| Slovenian | Rjava komatna tekica |
| Spanish | Canastera Común |
| Spanish (Spain) | Canastera común |
| Swedish | rödvingad vadarsvala |
| Thai | นกแอ่นทุ่งขอบปีกขาว |
| Turkish | Bataklıkkırlangıcı |
| Ukrainian | Дерихвіст лучний |
Glareola pratincola (Linnaeus, 1766)
Definitions
- GLAREOLA
- glareola
- pratincola
- Pratincola
The Key to Scientific Names
Legend Overview
Field Identification
22–25 cm; 60–98 g (1); wingspan 60–70 cm. Above brown , tinged olive, with white rump; long wingtips and deeply forked tail black; throat ochre-yellow, bordered narrowly with black; breast brown shading to white belly; underwing-coverts and axillaries deep rich chestnut; narrow but distinct white trailing edge to secondaries; bill red with black tip, legs blackish. In non-breeding plumage black border to throat indistinct, lores paler and breast mottled grey-brown. Differs from G. maldivarum in longer tail, contrast from above between darker outer and paler inner wing, and white trailing edge (although latter can be either absent or extremely difficult to see, especially in spring) (2); from G. nordmanni in last two features and also chestnut underwing. Juvenile mottled with black above and on breast; throat whitish without black collar. First-winter like non-breeding adult, but has broader pale fringes above and more streaks on head- and throat-sides. Races separated on small colour differences; Afrotropical breeders have deeper ochre thoat patch, more like G. maldivarum, obviously darker cap on crown and especially forehead, a long and contrasting mouth-line, generally darker upperparts (almost black on flight feathers), sometimes more deeply saturated (orange-buff) underparts, less extensive red underwing panel, occasionally a clear difference between pale inner and dark outer web of inner primaries, and a distinct purplish-green gloss to black feathers of upperparts (3).
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Closely related to G. maldivarum and G. nordmanni (although some range overlap with latter). Formerly considered to include G. maldivarum, and even on occasion G. nordmanni, as races. Has hybridized with G. nordmanni (4). Proposed form limbata (Red Sea coasts) included in nominate; possible race boweni of W Africa included in fuelleborni, as also is riparia (from S parts of African range). Species has on occasion been regarded as monotypic. Three subspecies recognized.
Subspecies
Glareola pratincola pratincola Scientific name definitions
Distribution
Glareola pratincola pratincola (Linnaeus, 1766)
Definitions
- GLAREOLA
- glareola
- pratincola
- Pratincola
The Key to Scientific Names
Legend Overview
Glareola pratincola erlangeri Scientific name definitions
Distribution
Glareola pratincola erlangeri Neumann, 1920
Definitions
- GLAREOLA
- glareola
- pratincola
- Pratincola
- erlangeri / erlangerii
The Key to Scientific Names
Legend Overview
Glareola pratincola fuelleborni Scientific name definitions
Distribution
Glareola pratincola fuelleborni Neumann, 1910
Definitions
- GLAREOLA
- glareola
- pratincola
- Pratincola
- fuelleborni / fuellebornii
The Key to Scientific Names
Legend Overview
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Flat and open areas, fields, steppe plains in Eurasia, usually near water; in Africa open ground, often recently burned, overgrazed grassland, ploughed fields, sand dunes, alkaline flats or sandflats, usually near water, especially along larger rivers and estuaries. Feeds over water, rice fields or coastal scrub; in SW Spain, preferentially over reed marshes and cereal croplands (5). On migration, occurs by water, and has been recorded to at least 3550 m in Himalayas of Ladakh (6) and 2880 m in Ethiopia (7).
Movement
Nominate race migrates after breeding to savannas of Sahel, where probably overlaps with birds of race fuelleborni in N tropics. Southbound passage migrant in Israel mainly in Aug and early Sept, moving N again in Apr and early May; common passage migrant through W Saudi Arabia, mainly around inland fresh waters; present in Pakistan Mar–Aug; some movement from Pakistan to W India, where species mingles with G. maldivarum; vagrancy recorded to most European countries N of breeding range (to Scandinavia, W to Madeira and Ireland). Many African populations nomadic, appearing and disappearing suddenly, often in response to changing water levels; may occur together with G. nordmanni; large numbers in Zambia in dry season, Apr–Nov. Flocks of 1000 or more birds regularly occur on migration; 30,000 birds noted moving N at Juba, S Sudan (04°52’ N) in early Apr, 1983, though not clear whether these were all Palearctic breeders, as this race thought not to winter so far S. Vagrant to Brazil (both offshore islands and the mainland) (8), Cape Verdes (9), Chagos Is (Diego Garcia) (10), Mongolia (11), China (as far E as Hong Kong) (12, 13), the Maldives (14) and Seychelles (12 records, late Sept–Nov, Jan) (15).
Diet and Foraging
Locusts and grasshoppers, beetles, termites, flies and other, mainly large, insects; also some spiders and molluscs. Research in S Spain that when feeding over cereal crops, the species predominantly takes the pests Aelia spp. and Eurygaster spp. (5) Forages in flocks, chiefly on the wing, catching aerial insects in graceful flight, often at dawn and dusk, sometimes on moonlit nights or where artificial light facilitates foraging (16); also chases prey with fast run on ground; leaps up to catch ephydrid flies along lake shoreline 10 cm above ground.
Sounds and Vocal Behavior
Mainly vocalizes in flight. Shrill, staccato and trilled calls, including a sharp, tern-like “stwick” in flight and a shrill, rolling trill that rises then falls in pitch, also given in flight, while at colony utters a shorter and more even-pitched trill in alarm, and in display flight a shrill “k’d’d’d’ik-kiik, kik”, with the first note downslurred (17). Loud twittering calls at nesting colonies can be audible over some distance (18).
Breeding
Lays May–Aug in Europe; May–Jun in Jordan; Apr–Jul Pakistan; in Africa, Mar–May in Sierra Leone, Mar–Jun in Ghana (19), Apr–Jun in Nigeria, Apr–May in S Ethiopia (7), Jun in S Somalia (20), Apr–Sept (wet season) in Kenya, Uganda and NE Tanzania, Jun–Sept (dry season) elsewhere in Tanzania after floodplains have dried out, Apr–Jun in N DR Congo, Jun–Nov in S DR Congo and Zambia (peak Aug–Oct in latter) (21), Aug–Oct in Zimbabwe and Malawi (22), Nov–Dec in Botswana, Aug–Dec in South Africa. Colonial nester in small groups of 10–20, up to 100 pairs, on dry mudflats or sandflats; sometimes forms mixed colonies with G. nordmanni and occasionally uses scrapes previously used by other birds, e.g. Kentish Plovers (Charadrius alexandrinus) (23). Seasonally monogamous. Nest usually a shallow scrape or natural depression in ground (7–9 cm wide by 4 cm deep) (18), such as a hoofprint, in Africa often next to piece of driftwood or plant, sometimes lined with dry plant fragments; each pair defends small nesting territory within colony, with 20–50 m between nests (24). Single-brooded. Usually three eggs (2–4) in Eurasia, 1–3 eggs in Africa (22), cream-coloured with black or dark brown markings, mean size 32·3 mm × 24·1 mm (25); incubation 17–19 days, by both sexes, starting with final egg (18). Chick mottled above with charcoal and black; below white. Young leave nest at 2–3 days; fed by both parents by regurgitation or by presentation of food in bill tip, up to age of seven days old; fledging 22–30 days (25). Comparison in Spain of breeding success in marshland and farmland found success much higher in former, despite equal amounts of insect food in both; however, egg losses during incubation much higher on farmland due to agricultural activities. Probably first breeding at one year old.
Conservation Status
Not globally threatened (Least Concern). Overall decline in range and numbers in Europe. Total of 6700–22,000 pairs breeding in Europe: largest populations are 250–1000 pairs in Russia (mid to late 1980s), 1000–1500 pairs in Greece, 500–5000 pairs in Turkey, c. 3800 pairs in Spain (fluctuating c. 3500–5000), and 250–1000 pairs in Portugal. During the period 2005 to 2013, just 45–127 pairs bred in France, where species first discovered nesting in 1941 (18, 26, 27). As many as 10,000 pairs estimated in Guadalquivir Marshes, S Spain, as recently as early 1960s; use of insecticides and herbicides caused reduction in population to c. 2250 pairs in 1990, though this is still the most important site in Europe, current threats being changes in water levels and human disturbance. In SE Europe, many sites of former breeding colonies destroyed through ploughing of grasslands, artificial irrigation and fertilization, use of pesticides, change in traditional grazing regimes, and commercial collecting of eggs; marked contraction of range noted in the former Yugoslavia, Hungary, Bulgaria and Romania (18). In addition to habitat loss and degradation through agriculture and flood control measures, increasing urban encroachment is also a threat, especially due to tourist pressure. Important colonies need protection, perhaps through establishment of reserves; measures also needed to reduce disturbance by agriculture and leisure activities. Decline in Ukraine from 3000 pairs in mid 1960s to 280–420 pairs in 1984; on N coast of Sea of Azov, Ukraine, colonization occurred in 1950s, replacing G. nordmanni; numbers fell sharply in 1970s and early 1980s; breeding success very low owing to nests being trampled by cattle and predated by Hooded Crows (Corvus corone cornix). Small numbers N of Caucasus; c. 500 pairs in Azerbaijan; range expansion further E in Chu Valley, Kyrgyzstan. Population of SW Asia numbers 10,000–100,000 pairs. Trends in E Palearctic not known. In N Africa, 200–500 pairs in Morocco, 200–250 pairs in Algeria, < 500 pairs in Tunisia, and > 1000 pairs in saltmarshes of Nile Delta, Egypt, with just one published breeding record in Libya (28). An occasional, and apparently opportunistic, breeder in Arabia, with confirmed records in Saudi Arabia and Oman, and a possible record in Bahrain (29, 24). Has become very uncommon as breeder in Israel, populations having declined by c. 90% since 1930s because of spraying with agricultural pesticides in species’ nesting habitat of cultivated fields; also some evidence of decline in neighbouring Jordan (30). Counts in sub-Saharan Africa include flocks of 3000 in Nigeria in Nov 1969; up to 100 together in S Somalia and 800 in Ethiopia (7); 1500 in rice fields to N of L Maga, Cameroon, in 1991; 10,000 present along just 5 km (10%) of shore of L Ngami, NW Botswana, Nov 1989, some nesting; 2000 near Salima, Malawi, Jan 1989 (22); several thousand throughout Jun 1993 along R Chobe, Caprivi Strip, border of NE Namibia and N Botswana; up to 3000 wintering on R Baro, Sudan, along with G. nordmanni. Numbers in Mali (both residents and Palearctic migrants) estimated at 10,000–50,000 individuals (31). Recently discovered breeding in coastal Kenya for first time (32). Widespread decline of this and other insectivorous species through increased use of pesticides, both in Europe and in Sahelian wintering grounds; also, water management projects in Sahel are altering habitat.
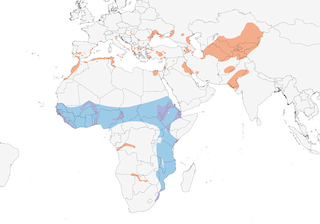
- Year-round
- Migration
- Breeding
- Non-Breeding































