Common Crane Grus grus Scientific name definitions
- LC Least Concern
- Names (49)
- Monotypic
Text last updated December 3, 2017
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Krilla |
| Arabic | كركي شائع |
| Armenian | Մոխրագույն կռունկ |
| Asturian | Grña comñn |
| Azerbaijani | Boz durna |
| Basque | Kurrilo arrunta |
| Bulgarian | Сив жерав |
| Catalan | grua europea |
| Chinese | 灰鶴 |
| Chinese (Hong Kong SAR China) | 灰鶴 |
| Chinese (SIM) | 灰鹤 |
| Croatian | ždral |
| Czech | jeřáb popelavý |
| Danish | Trane |
| Dutch | Kraanvogel |
| English | Common Crane |
| English (United States) | Common Crane |
| Faroese | Grátrani |
| Finnish | kurki |
| French | Grue cendrée |
| French (France) | Grue cendrée |
| Galician | Grou común |
| German | Kranich |
| Greek | (Ευρωπαϊκός) Γερανός |
| Gujarati | કુંજ |
| Hebrew | עגור אפור |
| Hungarian | Daru |
| Icelandic | Grátrana |
| Italian | Gru |
| Japanese | クロヅル |
| Korean | 검은목두루미 |
| Latvian | Dzērve |
| Lithuanian | Pilkoji gervė |
| Mongolian | Хархираа тогоруу |
| Norwegian | trane |
| Persian | درنای معمولی |
| Polish | żuraw |
| Portuguese (Portugal) | Grou-comum |
| Romanian | Cocor |
| Russian | Серый журавль |
| Serbian | Ždral |
| Slovak | žeriav popolavý |
| Slovenian | Žerjav |
| Spanish | Grulla Común |
| Spanish (Spain) | Grulla común |
| Swedish | trana |
| Thai | นกกระเรียนพันธุ์ยุโรป |
| Turkish | Turna |
| Ukrainian | Журавель сірий |
Grus grus (Linnaeus, 1758)
Definitions
- GRUS
- grus
The Key to Scientific Names
Legend Overview
Field Identification
c. 95–120 cm; male 5100–6100 g, female 4500–5900 g; wingspan 180–200 cm. Slate-grey body , with black primaries; dark head has white stripe starting behind eye and extending down nape; red skin patch on crown; iris yellow to orange. Similar to G. nigricollis, but has extensive white on head. Attains adult plumage in third year. Juvenile has crown feathered ; body plumage tipped with yellowish brown, upperparts have brown cast, no black tips to ‘bustle’ and flight feathers uniform and rather tapered. In second year attains adult-like head and body feathers, but crown not as bare, and tertials and wing-coverts may be still worn and brownish. Race archibaldi lacks red on crown.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Hybrids with G. monacha recorded on migration in NE China (1). New race archibaldi appears to be distinctive owing to absence of red on hindcrown, but other characters remain to be determined; not yet accepted by some authorities (2). E form lilfordi (Turkey to EC Asia; wintering NE Africa E to S China) sometimes recognized, but here regarded as probably undiagnosable, differences from nominate believed to be based on plumage colour variations due in part to differences in feather-painting behaviour. Two subspecies recognized.Subspecies
Hybridization
Hybrid Records and Media Contributed to eBird
-
Sandhill x Common Crane (hybrid) Antigone canadensis x Grus grus
-
Common x Hooded Crane (hybrid) Grus grus x monacha
Distribution
Scandinavia and NE Europe to NC China and Russian Far East; also Turkey and Caucasus, in Armenia and possibly Azerbaijan. Winters in France, Iberian Peninsula, NW & NE Africa, Middle East, Pakistan, India and S & E China.
Habitat
Nests in wide variety of shallow wetlands , including forested swamps (especially of birch and alder), sedge meadows and bogs. In Europe has adapted to smaller natural, artificial and restored wetlands. Throughout winter range, it forages in agricultural fields and pastures . Wintering habitats in W & SW Iberia distinctive, where species is characteristic of dehesas, open park-like areas chiefly consisting of widely spaced Iberian holm oaks (Quercus ilex) interspersed with pastures or crop stubble. Harvested ricefields also an important winter habitat in Spain (3). Everywhere, feeding is diurnal and flocks roost at nearby wetlands or other shallow waters. Undisturbed wetlands not impacted by hunting and sufficiently distant from human activities are primarily selected as roost-sites (4). However, in Libya large wintering flocks (300–1000+) have been found on irrigated fields in desert where there are no suitable wetlands and the birds are suspected to roost on open desert sands (5).
Movement
Major migration flyways well known. They comprise: from Scandinavia and N continental Europe through W Europe to wintering areas in France , Spain , Portugal and Morocco; from NE Europe through C Europe and Italy to wintering areas in Tunisia, Libya and Algeria; from E Europe and W Russia, through Balkans and across and around Black Sea to wintering areas in E Africa , Middle East and Turkey; from C Russia around Caspian Sea to SE Iraq and SW Iran; from W Siberia through Afghanistan and Pakistan to wintering areas in W & C India ; from N China and C Siberia across China to wintering areas along middle R Yangtze; and from Xinjiang and Qinghai provinces on Tibetan Plateau to wintering areas in S China. Migration is principally diurnal, often involving long flights between traditional staging sites, where large numbers may accumulate and rest for extended periods. Such staging sites include Hortobágy Plain in Hungary and Laguna de Gallocanta and La Sotonera reservoir, in E Spain (4) (6). Wintering locations in W Europe have shown some shift to N: in 1980/81 c. 100 individuals wintered in France, whereas in 2000/2001 there were c. 68,000 (7). Relatively few of those that enter Iberian Peninsula continue as far as Morocco, where there were only 500–2500 wintering birds in early 2000s (8). Iberia is the principal wintering area for W populations, especially those originating from Norway, Sweden and Germany, which winter in parts of C & SW Spain and SE Portugal (3). Wintering in E Spain has become progressively more frequent, increasing from 13% of Spanish wintering population in 1980 to 47% in 2007, largely due to short-stopping by young (9). Cranes enter Peninsula across its W half in concentrated passage that begins in Oct and peaks early Nov. However, recently they seem to have advanced their arrival at the Pyrenees by c. 20 days over a 30-year period, now peaking in mid to late Oct, perhaps reflecting earlier breeding in N Europe (10). Departure from Iberia chiefly evident at Pyrenees in late Feb and early Mar, this time in WC sector of range (6). Passage through Italy has increased markedly; in 2009, 36,934 birds were counted there. The birds involved have probably chiefly staged in the Hortobágy, from where a proportion cross the Adriatic and descend the Italian Peninsula, to enter Tunisia via Sicilian narrows. However, an increasing number cross N Italy and join cranes that winter in France and Iberia (11). Of those that cross the Sicilian narrows, > 20,000 have been found wintering on high Algerian Plateau post-2000, where numbers fluctuate annually, with some decline locally associated with land-use changes, temperature rises and human disturbance (12). Vagrants have reached Iceland (c. 40 records) and NE Greenland (Aug 1988). Birds found in North America may have been individuals that joined flocks of Antigone canadensis breeding in NE Asia and then migrated E; although some records from USA have been proven escapes, several from Alaska, the Great Plains and California are generally accepted as wild (13).
Diet and Foraging
Omnivorous. Plant items generally more important, especially outside breeding season: roots, rhizomes, tubers, stems, shoots, leaves, berries, seeds of emergent wetland plants, grasses, forbs and crop plants; also takes acorns, nuts, legumes and waste grain and rice. Animal items more frequent in summer diet: mainly invertebrates, including worms, snails, insects and other arthropods; also frogs, snakes, lizards, fish and rodents. Forages on land and in water by probing and picking.
Sounds and Vocal Behavior
Calls high-pitched, penetrating and far-carrying . Contact call, given mainly in flight, a deep, resounding, rolling “krro” or “karr”. Young in autumn give a piping “cheerp” call as well as adult-type calls. Breeding pairs often duet at dawn, the male giving a loud trumpeting call that female immediately follows with a lower-pitched “kraw” or sometimes a harsher “ka-ka-ka” (14).
Breeding
Spring breeder; most eggs laid in May. Nest a mound, c. 80 cm wide, of wetland vegetation; sited in shallow marsh or bog, often near trees. Usually two eggs; incubation 28–31 days; chicks dark brown above, pale brown below; fledging c. 65–70 days. Sexually mature at 4–6 years.
Conservation Status
Not globally threatened (Least Concern). CITES II. Global population estimated to number c. 491,000–503,000 individuals. Only European and European Russian populations are reliably surveyed on a regular basis and these totalled 113,000–185,000 pairs according to post-2000 counts (15). In Europe, largest subpopulations are those of Russia (25,000–40,000 pairs), Poland (20,000–22,000 pairs), Finland (23,000–50,000 pairs) and Sweden (21,000–39,000 pairs). The species as a whole is increasing, especiallyits W populations, although those elsewhere appear to be stable except some E populations which may be declining. Populations in much of the Europe are recovering after having been greatly reduced on eliminated during 19th and early 20th centuries. Extirpated as breeder in much of its historical range in S, W & E Europe: the last known instances of breeding were: in Italy c. 1880, Austria c. 1885, Hungary 1952, Spain 1954 (3) and Greece 1965. It bred in England until c. 1600 but has re-established a small population there, with nesting in Norfolk Broads since 1981 (16); a reintroduction project is ongoing in the Somerset Levels. British breeding population in 2015 numbered 26–32 breeding pairs that raised a total of 14 young and there were at least 12 non-breeding pairs; nesting or attempted nesting was reported from seven English counties and also two sites in Scotland, indicating a rapid spread (17). Small breeding population in Turkey and may breed in Azerbaijan, where until fairly recently it regularly overwintered at several sites. By 2005 c. 160,000 and 100,000 cranes were migrating on W European and Baltic–Hungarian routes, respectively, a three-fold increase since 1980s (7). Numbers have continued to increase since then. At L Der-Chantecoq, NE France, > 206,000 birds were counted in Nov 2014 Ornithological Note . Numbers wintering in Iberia have increased steadily. In Spain they numbered fewer than 20,000 in 1980, a large increase on the 4500 estimated there in ate 1950s; in 2007 151,243 were censused in Spain and more recent counts have exceeded 200,000 in Spain alone (3). In 2017, spring passage through the Pyrenees totalled 286,678 birds (18). Threats include loss and degradation of wetlands throughout breeding range, human pressures on wetlands in wintering grounds, changes in beneficial agricultural land uses and persecution intended to reduce crop damage. Hunting is of concern in some areas, particularly Afghanistan and Pakistan. Conservation efforts are mainly focused on W of range, coordinated through regular international meetings of European Crane Working Group. Key migration and wintering habitats protected in reserves and by agreements with landowners, especially in Germany, France and Spain. Populations are monitored closely in Europe and species is often included in waterbird surveys in other parts of range. The species is the subject of ringing and telemetry studies of migration routes in Europe, W Siberia and E Asia; extensive field studies in Europe, Russia, Ukraine, Pakistan, India , Nepal and China, and the focus of educational projects in W Europe, Pakistan, Russia and elsewhere.
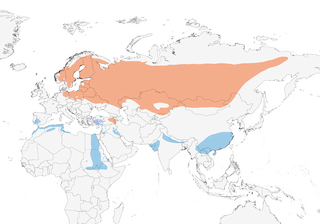
- Year-round
- Migration
- Breeding
- Non-Breeding




























