Little Black Cormorant Phalacrocorax sulcirostris Scientific name definitions
- LC Least Concern
- Names (25)
- Monotypic
Text last updated April 24, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Австралийски корморан |
| Catalan | corb marí negre |
| Czech | kormorán australský |
| Dutch | Zwarte Aalscholver |
| English | Little Black Cormorant |
| English (New Zealand) | Little Black Shag |
| English (United States) | Little Black Cormorant |
| Finnish | mustamerimetso |
| French | Cormoran noir |
| French (France) | Cormoran noir |
| German | Schwarzscharbe |
| Icelandic | Krákuskarfur |
| Indonesian | Pecuk-padi hitam |
| Japanese | ミナミクロヒメウ |
| Norwegian | lagfiskeskarv |
| Polish | kormoran bruzdodzioby |
| Portuguese (Portugal) | Corvo-marinho-preto-pequeno |
| Russian | Чёрный баклан |
| Serbian | Mali crni vranac |
| Slovak | kormorán ryhozobý |
| Spanish | Cormorán Totinegro |
| Spanish (Spain) | Cormorán totinegro |
| Swedish | sotskarv |
| Turkish | Kömür Karabatağı |
| Ukrainian | Баклан індонезійський |
Phalacrocorax sulcirostris (Brandt, 1837)
Definitions
- PHALACROCORAX
- phalacrocorax
- sulcirostris
The Key to Scientific Names
Legend Overview
Field Identification
55–65 cm; 520–1210 g; wingspan 95–105 cm. Rather long-tailed, small, slim, crestless cormorant with rather thin bill. Breeding adult has head, neck and most of body black with dull green to slightly purplish gloss, very short white filoplumes on head and neck, usually heavily concentrated on superciliary and crown-sides , with some longer filoplumes on neck, forming small white tuft just behind ear-coverts; tail and remiges duller blackish; mantle to upperwing secondary-coverts greyer, sometimes with purplish hue, each feather obviously fringed black giving scaled appearance, but often less well defined on greater coverts; white tuft on rear head soon lost during breeding season; non-breeding adult duller, tinged brown on head, neck and abdomen, indistinct and diffuse dull whitish line around gular pouch and base of bill; iris green; bare skin around eye, at base of mandible and on gular area blackish grey, can have lilac-blue tinge, gular skin fades to grey-blue in non-breeding season; bill darkish lead grey, little seasonal variation but may have horn tinge in non-breeding condition; legs blackish grey. Sexes similar. Juvenile entirely blackish brown, feathers of mantle to upperwing-coverts darker and more elongated than adult’s, especially on scapulars, and less contrastingly patterned, iris dull brown or dusky grey at first, soon becoming green, facial skin and gular pouch pale greyish or whitish with dark line over central lores, mandible also largely pale, while loral skin soon becomes dull brownish, bill and gular skin dull flesh-grey, dusky on culmen, subsquently plumage is browner , often mottled richest brown on foreneck and breast, perhaps with whitish on upper throat, and new feathers on mantle to upperwing-coverts more adult-like, when worn may look noticeably paler and greyer on head, neck and especially underparts. Closest to Nannopterum brasilianus, P. capensis and P. fuscicollis in size and structure; breeding adult readily separated from first by lack of white around gular skin, from first two by colour of naked skin on gape and gular region, and from third by lilac rather than blackish or dusky-yellow gular skin and by short white filoplumes largely covering superciliary region, while post-auricular tuft of longer plumes is usually reduced and soon lost; young birds more tricky, but naked gape and gular skin of present species grey or very dull flesh instead of yellowish to orange, and also longer-tailed than P. capensis, the bare gular skin extending on midline as far as gape (less extensive in Nannopterum brasilianus), while P. fuscicollis further differs by often whitish throat; the usually weaker bill separates juvenile of present species from that of P. fuscescens and larger P. varius.
Systematics History
Subspecies
Distribution
Java and Wallacea E to New Guinea, Aru Is, Australia, Tasmania, New Zealand (mostly North I) and New Caledonia (1).
Habitat
Mainly inland waters , including lakes and swamps; commonly on reservoirs and ponds, even in urban parks; also rivers and flood channels. Less frequent in sheltered coastal waters, including estuaries, inlets, lagoons, mangroves , salt pans, etc. Inland, prefers open waters more than 1 m deep. Roosts on trees, bushes and rocks.
Movement
Mainly sedentary. Dispersive in Australia, with major movements related to droughts and temporary flooding; more common along coast when drought conditions prevail inland. In New Zealand may migrate to coastal waters of N for winter. Some movement between Australia and New Zealand, and also between islands of Indonesia. This rather nomadic species appears to be an irregular visitor to New Britain, Bougainville and New Caledonia (2). Vagrant to Lord Howe I and Norfolk I. One at Nacomoto, in SE Kadavu I, in Nov 2004 had, according to villagers, been present for more than one year, and was apparently the first record in Fiji (3).
Diet and Foraging
Mainly fish; also some crustaceans and other invertebrates. In much of Australia takes large quantities of introduced fish, e.g. carp (Carassius auratus) and perch (Perca fluviatilis). On Magela floodplain, in N Australia, takes almost exclusively fish, mostly less than 4 cm in length, especially the plotosid catfish Neosilurus rendahli, sailfin perchlet (Ambassis agrammus) and Melanotaenia splendida; in estuaries of SW Australia mainly Attherinidae and Gobiidae species. Feeds mainly by pursuit-diving . Frequently fishes co-operatively, sometimes with more than 1000 birds together, efficiency increasing with number of birds; crayfish captured individually.
Sounds and Vocal Behavior
At breeding site, displaying male gives hoarse bark and “ak-he” call; harsh croaking as threat; repeated ticking sounds before movement, also loud “krah”; also “tu tu” whistle during interactions between partners. Various bickering calls at roost.
Breeding
Breeds in all months, depending on water conditions and food availability; Apr–Aug in N Australia. Normally forms small colonies of few pairs, generally fewer than 100, but occasionally more than 1000 recorded; often in company of other cormorants, darters, egrets, herons or ibises. Nest made from twigs and reeds, lined with leaves, grass and bark, placed in high fork in tree, often over water, or in bush. Clutch up to six eggs , normally 3–4; no information on incubation period; chicks naked, grow black down .
Conservation Status
Not globally threatened (Least Concern). Widespread in Australia, with breeding colonies of up to 1400 nests reported. Has benefited in Australia from construction of dams and reservoirs, with increased habitat for both foraging and breeding. Less affected than most other species by transformation of wetlands, as it uses mainly deeper waters and estuaries; some breeding grounds threatened by increased salinity, clearing, grazing, extraction of groundwater and burning. Feeds extensively on introduced fish species, and campaigns to remove such fish could have negative effects on this species. A rare breeder in and very common visitor to lowlands of New Guinea; partial census in Jan 1990 gave 1714 in Papua New Guinea. Locally common in Sulawesi. Spread W to Java during 20th century. Spread S in New Zealand, where first breeding record in South I in 2008 near Christchurch (4). Locally common in Lesser Sundas ; uncommon on Damar I (Banda Sea), where two observations of singles in 2001, one at an estuary near Batumerah in Aug and the other in flight near Kumur in Sept (5).
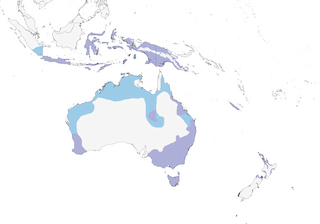
- Year-round
- Migration
- Breeding
- Non-Breeding