Red-breasted Flycatcher Ficedula parva Scientific name definitions
- LC Least Concern
- Names (51)
- Monotypic
Text last updated February 20, 2019
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Mizakapësi gushëkuq |
| Arabic | خاطف الذباب أحمر الصدر |
| Armenian | Փոքր ճանճորս |
| Asturian | Mosqueru pequeñu |
| Azerbaijani | Xırda milçəkqapan |
| Basque | Euli-txori papargorria |
| Bulgarian | Червеногуша мухоловка |
| Catalan | papamosques menut |
| Chinese | 紅胸鶲 |
| Chinese (Hong Kong SAR China) | 紅胸姬鶲 |
| Chinese (SIM) | 红胸姬鹟 |
| Croatian | mala muharica |
| Czech | lejsek malý |
| Danish | Lille Fluesnapper |
| Dutch | Kleine Vliegenvanger |
| English | Red-breasted Flycatcher |
| English (United States) | Red-breasted Flycatcher |
| Faroese | Smánápur |
| Finnish | pikkusieppo |
| French | Gobemouche nain |
| French (France) | Gobemouche nain |
| Galician | Papamoscas real |
| German | Zwergschnäpper |
| Greek | (Ευρωπαϊκός) Νανομυγοχάφτης |
| Hebrew | חטפית גמדית |
| Hungarian | Kis légykapó |
| Icelandic | Peðgrípur |
| Italian | Pigliamosche pettirosso |
| Japanese | ニシオジロビタキ |
| Kannada | ಕೆಂಪುಎದೆಯ ನೊಣಹಿಡುಕ |
| Korean | 서양흰꼬리딱새 |
| Latvian | Mazais mušķērājs |
| Lithuanian | Mažoji musinukė |
| Malayalam | ചെമ്മാറൻ പാറ്റപിടിയൻ |
| Marathi | लाल छातीची माशीमार |
| Norwegian | dvergfluesnapper |
| Persian | مگس گیر سینه سرخ |
| Polish | muchołówka mała |
| Portuguese (Portugal) | Papa-moscas-real |
| Punjabi (India) | ਲਾਲ-ਹਿੱਕੀ ਟਿਕਟਿਕੀ |
| Romanian | Muscar mic |
| Russian | Малая мухоловка |
| Serbian | Mala muharica |
| Slovak | muchárik malý |
| Slovenian | Mali muhar |
| Spanish | Papamoscas Papirrojo |
| Spanish (Spain) | Papamoscas papirrojo |
| Swedish | mindre flugsnappare |
| Thai | นกจับแมลงพันธุ์ยุโรป |
| Turkish | Küçük Sinekkapan |
| Ukrainian | Мухоловка мала |
Ficedula parva (Bechstein, 1792)
Definitions
- FICEDULA
- ficedula
- parva
The Key to Scientific Names
Legend Overview
Field Identification
11·5 cm; 8·5–11·5 g. Male breeding has forehead, crown and hindneck brown, tinged grey, lores, ear-coverts and neck-side ashy grey, narrow eyering off-white; upperparts , including upperwing, brown, flight-feathers and upperwing-coverts narrowly edged paler brown, uppertail-coverts blackish brown, tipped grey-brown; tail blackish brown, T3 with middle third of outer web white (often extending to middle of inner web), outer three feather pairs (T4–T6) with basal two-thirds of both webs white; chin, throat and breast orange-red (becoming brighter with age); side of upper breast grey, rest of underparts white, tinged creamy buff on flanks and side of lower breast, thighs pale brownish; axillaries and underwing-coverts creamy buff; iris brown; maxilla blackish horn, mandible yellowish to deep flesh-pink, variably dark towards tip; legs dark brown. Distinguished from F. subrubra mainly by paler coloration, less extensive and paler red below, no blackish border on upper breast side. Non-breeding male has less extensive orange-red on chin and throat, and flanks more buffish. Female has forehead, crown and hindneck brown (with no grey tinge), lores buffish white, ear-coverts pale brown, narrow eyering buffish, upperparts and upperwing as male, with blackish uppertail-coverts, chin, throat, breast side and flanks creamy buff, rest of underparts white. Juvenile has lores, ear-coverts and upperparts rufous-buff with narrow dark brown feather edges, giving spotted appearance, underparts buff, deeper and yellower on breast and flanks, belly and undertail-coverts white; immature as female, but inner secondaries narrowly edged rufous-buff, and tertials, greater coverts and outer median coverts edged and tipped rufous-buff.
Systematics History
Closely related to F. subrubra; sometimes considered conspecific, but the two differ distinctly in plumage and wing structure. Until recently, considered conspecific with F. albicilla (which see). Monotypic.
Subspecies
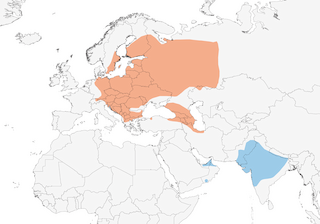
Distribution
Habitat
Breeds in forest and woodland, mainly mixed deciduous stands, especially with beech (Fagus), but also in oak (Quercus) forest; also in spruce (Picea) forest in N of range. Prefers tall trees with much undergrowth, light canopy, and an open zone with perching twigs between canopy and undergrowth layer; favours glades, clearings and areas near water. Also occurs in orchards and vineyards. In Austria, territories were correlated positively with the numbers of hornbeams (Carpinus betulus), the age of the stands and volume of lying dead trunks, and were sited mainly found in stands with high levels of mammalian game activity and accordingly a more sparse bush layer and less ground cover; the species also avoided areas that were intensively managed. Breeds in lowlands and hills, to 2350 m in Caucasus. In non-breeding areas occurs in groves, forest plantations, open woodland, forest edge, secondary growth, parks, orchards, and urban gardens with large trees; to at least 2100 m. On passage also in scrub; recorded on passage in N Africa (where scarce) in woodland, plantations and gardens, often in large trees, but also in taller bushes and thickets in drier country, and at Saharan oases.
Movement
Long-distance migrant. Most individuals migrate SE to non-breeding grounds in Pakistan and India , but a few (presumably from W populations) regularly move across E Mediterranean and NE Africa from early Sept to Dec; winters irregularly in small numbers also in S Caspian region, Afghanistan, Sinai, Arabian Gulf states and Oman ; in autumn occurs also W & SW of breeding areas, with Oct–Jan records S to Morocco, Algeria, Tunisia and N Senegal, and a few possibly winter regularly S of Sahara in areas of W Africa. Migrates alone or in small flocks. Autumn movement protracted, with three main routes to India and Pakistan: S of Black Sea across Balkans, Bosphorus and N Turkey; N of Black Sea across Caucasus into Iran; and S along Urals and E of Caspian, through W Kazakhstan and Tajikistan. Departure from Europe begins late Jul, is most pronounced Aug to mid Sept, continuing to early Oct, Russian birds leaving mid Aug to end Nov; passage through Iran end Sept to early Nov, and main passage through Afghanistan in Oct; arrives in Pakistan and rest of winter quarters from late Aug to Nov, mainly Sept–Oct. Return passage begins mid Mar, peak in Apr and continuing into May; arrivals in Caucasus from early Mar, but main passage there, on Black Sea coast and in Turkey mid Apr to early May; reaches Russian breeding grounds Apr–May, and C Europe late Apr to early Jun; occasional spring records in E Libya and Egypt mid Apr to early May. Vagrant/rare visitor in China, Japan, Iceland, Faeroes, France , Spain (including Canary Is), Portugal , Britain (almost 5000 records between 1968 and 2016) and Switzerland (in W Europe mainly in autumn, to lesser extent spring, exceptionally winter).
Diet and Foraging
Eats mainly insects and other invertebrates, especially beetles (Coleoptera) and spiders (Araneae), but also dragonflies and damselflies (Odonata), stoneflies (Plecoptera), grasshoppers (Orthoptera), earwigs (Dermaptera), bugs (Hemiptera), adult and larval lepidopterans, adult and larval flies (Diptera), ants and wasps (Hymenoptera), harvestmen (Opiliones), earthworms (Oligochaeta), woodlice (Isopoda) and snails (Gastropoda). Usually solitary ; often skulks in canopy or in bushes. Hunts mainly from middle to lower layers, hopping and creeping among foliage, and sometimes hovering like a Phylloscopus warbler. Makes short, looping sallies to catch prey in the air, with agile flight and rapid wingbeats; frequently forages from the ground, including among grass. In damp places, takes aquatic invertebrates, such as larval water beetles (Dytiscidae). Regularly regurgitates pellets. Often flicks wings, and cocks and flirts tail.
Sounds and Vocal Behavior
Song melodious, resonant and loud, characterized by whistling notes on descending scale, notes variously rendered “tui”, “dlu”, “didle”, “diu-tvi-diu-tvi” and similar, often likened to song of Willow Warbler (Phylloscopus trochilus) but also not unlike that of F. hypoleuca; song often preceded by “zit” calls. Call a thin, high-pitched, short “dzik” or “zit”, sometimes protracted into loose ticking “tk tk tk…” series; also rattling “zrrrt” of alarm and plaintive “hveet”.
Breeding
Nests mid May to end Jun in C & E Europe and in late May and Jun in former USSR; usually single-brooded. Monogamous; solitary nester. Nest built by female, in 3–5 days, a cup of moss, dry grass stalks and leaves, root fibres and hair, lined with hair, sometimes lichen woven into outside, external diameter 84–100 mm, depth 48–65 mm, inner diameter 50–52 mm, depth 25–45 mm; placed usually 1–3 m (sometimes 4 m or higher, up to 21 m recorded) above ground in hole in tree or wall, among side shoots of trunk, sometimes on branch close to trunk, or even fork in branches, and nestbox also accepted; occasionally located in bush (nest may then be domed); territory 0·5–0·7 ha in optimal habitat in Belarus. Clutch 4–7 (usually 5–6) whitish, sometimes faintly buff or blue-green eggs with fine reddish-brown markings typically concentrated at larger end, mean size 16·5 mm × 12·6 mm, laid at daily intervals; incubation by female alone (provisioned by male), usually from last egg, or 1–2 days before last egg laid, period 12–15 days (usually 12–13 days); chicks brooded by female, fed by both parents, nestling period 11–15 days (usually 12–13 days); young fed for at least 4–5 days after fledging, independent by 27–29 days of age. Of 96 eggs in 18 nests in E Germany, 34% produced fledglings; in Belarus, 54% of eggs laid resulted in fledged young, with most losses due to predation; in NE Poland, young fledged successfully from 51% of nests, and 82% of unsuccessful clutches were lost to predators (mostly during egg-laying and the later incubation periods). Studies in NE Poland have found that (1) the size of the red breast patch and earlier arrival time on the breeding grounds positively influenced mating success for males, (2) more than 70% of clutches hatched asynchronously (with the highest percentages in years when breeding commenced later), and (3) clutch size did not depend on type of nest and breeding success was influenced by height of the hole above ground and its depth; success was similar in all types of nest-sites, and no differences were observed in breeding success in dead or live trees or different hole orientation, thus depth of the hole seems to be the most important single factor determining whether a brood survives or not. Although individual broods range from all male to all female, mean offspring sex ratio at the population level in NE Poland does not deviate significantly from 50:50 sex; however, females mated to older males (which typically arrive earlier on the breeding grounds) were significantly more likely to produce male-biased broods, suggesting that females manipulate sex ratio in response to their mate’s age.
Conservation Status
Not globally threatened (Least Concern). Common. In Europe, scarce in W of range but becomes commoner to E, reaching highest densities in Slovakia, Poland, Belarus, the Baltic countries and Romania. European population estimated at c. 343,000 pairs, with additional c. 3,160,000 pairs in Russia and 3150 in Turkey. Breeding density reaches 0·7 (mean 0·28) pairs/10 ha in N Germany, and is 0·6–2 (mean 1·2) pairs/10 ha in Poland; in Belarus as high as 61–124 pairs/km², but in Moscow region density only 8–25 pairs/km². Population and range in Europe remained fairly stable during 1970–1990, although range contracted and numbers decreased in Ukraine, numbers fell in Finland, Lithuania and Austria, and numbers increased in Netherlands, Czech Republic and Slovenia. German population currently estimated at 1400–2200 pairs (data for 2005–2009), where numbers estimated to have declined since the late 1990s. The species’ reliance on old, diversified forests makes it vulnerable to modern forestry practices in Europe, including shorter rotations and the felling of old trees; this suggests that it may face a shortage of nesting sites.
- Year-round
- Migration
- Breeding
- Non-Breeding































