Ruby-topaz Hummingbird Chrysolampis mosquitus Scientific name definitions
- LC Least Concern
- Names (26)
- Monotypic
Text last updated February 19, 2013
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | colibrí robí |
| Czech | kolibřík červenohlavý |
| Dutch | Muskietkolibrie |
| English | Ruby-topaz Hummingbird |
| English (United States) | Ruby-topaz Hummingbird |
| French | Colibri rubis-topaze |
| French (France) | Colibri rubis-topaze |
| German | Moskitokolibri |
| Japanese | ルビートパーズハチドリ |
| Norwegian | rubintopas |
| Polish | moskitnik |
| Portuguese (Brazil) | beija-flor-vermelho |
| Portuguese (Portugal) | Beija-flor-vermelho |
| Russian | Колибри-москит |
| Serbian | Rubin-topaz kolibri |
| Slovak | jagavička rubínová |
| Spanish | Colibrí Rubí |
| Spanish (Argentina) | Picaflor Topacio |
| Spanish (Panama) | Topacio Rubí |
| Spanish (Paraguay) | Colibrí rubí |
| Spanish (Peru) | Colibrí Rubí-topacio |
| Spanish (Spain) | Colibrí rubí |
| Spanish (Venezuela) | Tucusito Rubí |
| Swedish | topaskolibri |
| Turkish | Topaz Taçlı Altıngerdan |
| Ukrainian | Колібрі-рубін |
Chrysolampis mosquitus (Linnaeus, 1758)
Definitions
- CHRYSOLAMPIS
- mosquitus
The Key to Scientific Names
Legend Overview
Introduction
The Ruby-topaz Hummingbird is a much coveted gem of circum-Amazonian savanna habitats from Colombia east through Venezuela, the Guianas, south through Brazil and west to eastern Bolivia. It is a very small hummingbird, but with a brilliant ruby crown and nape, iridescent gold throat and breast and bright orange tail and is luckily, quite common throughout its range. It forages for the nectar of flowering shrubs from the understory to tree tops in open country but also in cultivated areas and gardens.
Field Identification
8–9·5 cm (1); 2·5–5 g (2, 1). Male has short straight black bill; crown and nape shining ruby red (occasionally orange), back dark brown glossed dull olive; throat and breast iridescent golden (occasionally emerald-green ); rest of underparts brown ; tail rich chestnut tipped black . Female has copper-green back ; below pale grey (on both Trinidad and Tobago, some birds have greenish-golden stripe from chin to breast); central tail feathers olive-green, the others rufous-chestnut with shining purple-black subterminal band, tipped white; bill averages longer than that of male (3). Juvenile similar to adult female; has white spot behind eye, outer tail dark violet, tipped white . Non-adult male plumages might be easily confused with other hummingbirds, but for distinctive and unusual head shape, as well as presence of rufous in outer tail feathers (3).
Systematics History
Subspecies
Distribution
E Panama and W, N & C Colombia E through Venezuela to the Guianas, then S through NE & C Brazil (Pará to Pernambuco and S to Mato Grosso) to E Bolivia; also islands off N Venezuela coast from Aruba, Curaçao and Bonaire E to Trinidad and Tobago.
Habitat
Occurs in savanna-like vegetation (including campos rupestres) (5) and its edges (6) from sea-level to shrubby arid hillsides at 1700 m, with migrants even recorded on river islands (6); forages from low down to treetops in clearings, open country, gardens and cultivated areas. Most numerous below 500 m; birds at higher altitudes are currently not breeding.
Movement
Migratory. Arrives in S Cauca Valley, Colombia, in May and disappears in Sept; absent or rare in Trinidad & Tobago in Aug–Nov and has wandered N to Grenada (Sept 1962) and perhaps even the Grenadines; appears in Paraná, Brazil (where occasionally recorded on inshore islands) (7), in Oct and leaves in Apr, in which latter month there have been several recent records (since 2001) in NE Argentina (Misiones) (7), and is considered solely a wet-season visitor (Jan–May) to the Chapada Diamantina, Bahia (Brazil) (5); however, temporal status at some localities in Pernambuco is unclear (8). Overall, within Brazil, N–S migration, with most records in Amazonia apparently referring to migrants, e.g. in Jaú National Park and the INPA reserves N of Manaus, on N bank of R Negro and Solimões, and the Serra dos Carajás, S of lower Amazon, with a recent (Apr) record from Acre, although an apparently resident population is present on the lower R Aracá, Amazonas (9); along the coastal areas of the Guianas, Venezuela and Colombia presumably an E–W migration with southward tendency towards Cauca and Magdalena Valleys, Colombia. Recent (documented) records from N Bolivia (in Nov and Apr) are also strongly suggestive of migration (6).
Diet and Foraging
Nectar of flowering shrubs, cultivated crops, cacti , small and large trees: Russelia, Cajanus, Isertia, Melocactus, Citharexylum, Samanea, Cordia, Palicourea (10), Sarcopera (11), Lantana (11) and Inga (6), as well as at least one liana, Arrabidaea (12). In Bahia, E Brazil, in a campo rupestre, this species was observed feeding on the following plants: Hohenbergia ramageana (Bromeliaceae), Camptosema coriaceum, Periandra mediterranea (Fabaceae), Prepusa montana (Gentianaceae), Humiria balsamifera (Humiriaceae), Cuphea ericoides (Lythraceae), Calliandra mucugeana, C. viscidula (Mimosaceae), Stachytarpheta crassifolia (Verbenaceae) and Vochysia pyramidalis (Vochysiaceae) (5), while at another locality, in the caatinga of Pernambuco, C. mosquitus was observed feeding at flowers of Tabebuia impetiginosa (Bignoniaceae), Tacinga palmadora (Cactaceae), Cnidoscolus halteris, Jatropha mollissima (Euphorbiaceae), Bauhinia cheilantha (Fabaceae) and Melochia tomentosa (Malvaceae) (8); also Pavonia glazioviana (Malvaceae) in caatinga of Piauí (13). In one study, aggressive interactions at flowers involving C. mosquitus were mainly directed at conspecifics, rather than other species of hummingbirds (5), but at least locally dominates Chlorostilbon lucidus (8). Small insects (e.g. Areneae) (14) are caught in the air by hawking. Occasionally seen foraging for arthropods among foliage along clearings and roads. Male defends feeding territories in flowering shrubs or trees.
Sounds and Vocal Behavior
Song (given from high perch) is reportedly a high-pitched, doubled “tliii...tliii...tliii” (3).
Breeding
Season Dec–Jun on Trinidad and Tobago, Venezuela, Guianas; Sept–Mar in Brazil. Tiny cup-shaped nest of fine plant fibre and cobweb, outside decorated with lichen and/or pieces of bark, is built in fork of small branch or on a branch (1), 1–4 m above ground, occasionally up to 8 m; nest height 30 mm, external diameter 40 mm, internal diameter 25 mm (1). Clutch size two white eggs , size 11·8–14·2 mm × 8·4–9 mm, mass 0·45 g (2, 1); incubation 15–16 days, by female; chick black with sparse brownish dorsal down; fledging in 19–22 days (although period as long as 28 days mentioned in some literature) (1). First breeding in second year.
Conservation Status
Not globally threatened (Least Concern). CITES II. Common resident in the lowlands and coastal ranges, with densities of at least 6–8 pairs/km² in shrub-like habitats of SW Trinidad. Readily accepts man-made habitats like gardens and cultivated areas. Present in Tayrona National Park (Colombia) and Serra da Capivara National Park (NE Brazil) (13). Until 1970 the most sought-after species of hummingbird for the international bird trade in Brazil; this has now been ended.
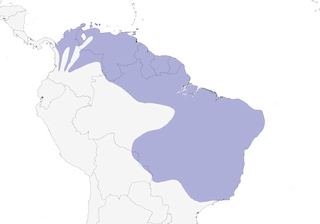
- Year-round
- Migration
- Breeding
- Non-Breeding