Short-tailed Shearwater Ardenna tenuirostris Scientific name definitions
- LC Least Concern
- Names (36)
- Monotypic
Text last updated February 1, 2015
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Kortstertpylstormvoël |
| Catalan | baldriga de Tasmània |
| Chinese | 短尾水薙鳥 |
| Chinese (Hong Kong SAR China) | 短尾鸌 |
| Chinese (SIM) | 短尾鹱 |
| Croatian | tankokljuni zovoj |
| Czech | buřňák tenkozobý |
| Dutch | Dunbekpijlstormvogel |
| English | Short-tailed Shearwater |
| English (United States) | Short-tailed Shearwater |
| French | Puffin à bec grêle |
| French (France) | Puffin à bec grêle |
| German | Kurzschwanz-Sturmtaucher |
| Hebrew | יסעור קצר-זנב |
| Hungarian | Vékonycsőrű vészmadár |
| Icelandic | Eyjaskrofa |
| Indonesian | Penggunting-laut ekor-pendek |
| Japanese | ハシボソミズナギドリ |
| Korean | 쇠부리슴새 |
| Malayalam | കുറുവാലൻ തിരവെട്ടി |
| Norwegian | smalnebblire |
| Polish | burzyk cienkodzioby |
| Portuguese (Brazil) | pardela-de-cauda-curta |
| Portuguese (Portugal) | Pardela-de-cauda-curta |
| Russian | Тонкоклювый буревестник |
| Serbian | Kratkorepi zovoj |
| Slovak | víchrovník tenkozobý |
| Slovenian | Kratkorepi viharnik |
| Spanish | Pardela de Tasmania |
| Spanish (Costa Rica) | Pardela Colicorta |
| Spanish (Mexico) | Pardela Cola Corta |
| Spanish (Spain) | Pardela de Tasmania |
| Swedish | kortstjärtad lira |
| Thai | นกจมูกหลอดหางสั้น |
| Turkish | İnce Gagalı Yelkovan |
| Ukrainian | Буревісник тонкодзьобий |
Ardenna tenuirostris (Temminck, 1836)
Definitions
- ARDENNA
- tenuirostra / tenuirostre / tenuirostris
The Key to Scientific Names
Legend Overview
Introduction
The Short-tailed Shearwater exclusively breeds on islands off southern and eastern Australia, where the species’ chicks (known as muttonbirds) are extensively harvested under license from the government. However, during the non-breeding season (from late April to mid September), the species performs one of the most remarkable of trans-equatorial bird migrations, with the entire population (estimated at perhaps as many as 30 million individuals) moving north across the central Pacific to the Bering Sea, where the birds pass the boreal summer, before they start to move south again in August, following a more easterly track on the return journey. Some individuals even reach the Arctic Ocean during this period. The Short-tailed Shearwater is gregarious at sea, as well as on land, and feeds mainly underwater, diving up to 70 m below the surface in pursuit of small fish, crustacea and cephalopods. Despite its English and scientific names, neither is this species’ tail especially short in relation to other shearwaters, nor is the bill particularly slender.
Field Identification
40–45 cm; 480–930 g (1); wingspan 91–100 cm (2). Cap to below eye dark sooty-brown or dark sooty-grey, becoming slightly paler over rest of upperparts, darker again on remiges , tail and rear scapulars, browner feather fringes affording a slight scaly appearance at close range, especially on scapulars; usually paler on underparts thus head often looks capped; underwing variable, has narrow and usually diffuse dark margin, often broadest on distal secondaries, axillaries and rest of wing-coverts and primary bases rather plain brownish except paler bar on centre of inner wing, some have nearly plain brownish underwing, palest birds decidedly paler buffy-grey on parts of coverts, outermost median primary-coverts may have darker brown shaft-streaks, and primary bases also broadly pale on such birds; iris blackish; bill brownish horn to dark grey, often darker near tip; legs and feet dull pink-flesh to brownish grey, with variable dusky tinge. Sexes alike, but males on average larger, especially in bill depth and head length (1). Juvenile as adult, but fresh in May–Jul when older birds are heavily worn or in moult, while presumed first-cycle birds in Sept–Nov (at least) can have contrasting dark head and faded underparts (2). Sometimes looks notably stubby and small-winged , but most similar in colour and structure to A. grisea, but underwing plainer brownish or has pale area on distal wing less white but much larger and diffuse, usually lacking sharply contrasting shaft-streaks, and not contrasting with primary bases and greater coverts, also has more contrasting pale throat (in some birds), externallink (⅓ or less in A. grisea). Puffinus nativitatis has broader-tipped wing, more slender body, proportionately longer and blacker bill, darker legs hardly projecting beyond longer tail, and is entirely dark chocolate-brown, with no contrast on throat or underwing.
Systematics History
Subspecies
Distribution
Pacific and S Indian Oceans, breeding in Tasmania and S Australia (bulk of population in SE).
Habitat
Marine ; occurring in inshore, offshore and, to lesser degree, pelagic waters. Breeds mainly on coastal islands (but also mainland headlands) (3), typically in areas of grassland or other vegetation , but sometimes on cliffs or bare ground, and appears to be something of a generalist compared to other burrow-nesting seabirds on Australian islands (4).
Movement
Transequatorial migrant. During breeding season also wanders over large areas of ocean as, although especially abundant off SE Australia and in Tasman Sea, also regularly visits Antarctic waters, mostly within 30–80 km of ice edge, with records from at least 84º S (5), mainly during pre-laying exodus and when feeding chicks (3). Post-breeding most birds (immatures first in Mar, adults in mid Apr and fledglings in late Apr and early May) (3) initially move E then NW, passing both E & W of Fiji and past Japan to reach Sea of Okhotsk and W end of Aleutian Is by mid to late Apr (but mostly May/Jun), some moving N of Bering Strait, even reaching polar zones off Russian Wrangel I (3) and Chukchi Sea (late Jul to mid Nov) (2); however, recent satellite-tracking work has found that an adult initially returned to Antarctic waters, presumably to feed, prior to commencing its transequatorial migration (6), while regular passage in comparatively small numbers between late Apr and late May has recently been noted off Hong Kong (7). Return route over C Pacific commences in Aug, some moving down W coast of North America (although can arrive off Alaska and British Columbia as early as late Apr and even remain to late Feb) (8), with generally smaller numbers off Washington to C California (mainly Oct–Dec, exceptionally from late Jul and can linger even until Apr/May) and only very rare S to NW Mexico (as far as Guerrero, Oct 1972) and an unconfirmed record from Costa Rica (2); reaches E Australia by late Sept (3). More recently, geolocators have been used to track birds from a colony on Great Dog I, off Tasmania: post-breeding, they flew S of the Antarctic Polar Front to a previously unknown stopover site, where they remained for several weeks, before travelling rapidly N through the W Pacific Ocean to waters off Japan, spending the bulk of the boreal summer either there or further N in Bering Sea, before returning S through C Pacific to their breeding sites (9). There is also apparently some regular movement into N Indian Ocean, with W–E passage through Singapore Strait in May (10), increasingly frequent records from W coast of Thailand and N Malaysia (exceptionally present as late as end of Jun) (11, 12), older specimens from Pakistan and Sri Lanka, more recent sight records from Sri Lanka and the Maldives, in Nov 2003 (13), photographic records from Bangladesh (Apr 2008) (14) and mouth of Ganges R, West Bengal (Apr 2013) (15, 16), and a moribund individual on island of Rodrigues, in SW Indian Ocean (Jun 1974) (17). Even more exceptional instances of vagrancy in Atlantic, with sight record off Virginia (USA) in Jan 1998, a moribund bird off Florida in Jul 2000 (2), and a beached specimen in Bahia, Brazil, in May 2005 (18).
Diet and Foraging
Fish (Mallotus villosus, Ammodytes hexapterus, Sardinops sagax, Trachurus declivis, Engraulis australis) (19), crustaceans (Nyctiphanes australis, Thyssanoessa raschii, Calanus cristatus, Brachyura sp.) (19) and cephalopods (Nototodarus gouldi, Sepioteuthis australis) (19); proportions vary with season and locality, e.g. pre-laying season diet (Sept/Oct) dominated by crustacea (80%), but between Feb and Apr, when feeding chicks, crustacea form only c. 40% of diet, with fish found in 80% of samples during such periods, although those feeding in Antarctic waters at such times mainly take Euphausia superba, while in non-breeding season balance between different constituents tends to be less weighted (3). Feeds mainly by deep-plunging (20), pursuit-plunging and surface-diving, from up to 3–5 m above sea (2), frequently reaching 20 m below surface, exceptionally 70 m (3); other techniques include surface-seizing and hydroplaning. More prone to follow and scavenge behind boats than P. griseus (2). Feeds in flocks of up to 20,000 birds, often with other seabirds, including other shearwaters, gulls, cormorants and terns (20, 2), and displays a degree of foraging site fidelity (21). Associates with cetaceans (e.g. humpback whales Megaptera novaeangliae) (2).
Sounds and Vocal Behavior
Like A. grisea is noisy at colonies but primarily calls from ground, not in flight, and principal call (in burrow defence) is mixture of inhalation and exhalation, rendered “ee-ee-a-aa”, perhaps slightly higher-pitched in male than in female, also like A. grisea; during copulation gives distinctive “crukcrukcrukcrukcruk crooer crukcruk...” (3).
Breeding
Starts Oct, though arrives at colonies from mid Sept, and these are fully occupied by 25 Sept, with pre-laying exodus (sometimes complete, at least in Tasmania) of 20–21 days, egg-laying last week of Nov (mean 25–26 Nov, little variation between colonies or years and 85% of eggs laid within three days of mean), hatching around mid Jan and fledging in late Apr (3). Long-term monogamous (genetic study identified eight young in 83 that were product of extra-pair matings) (22), with one study recording that half of all males and females had only one partner during their lives (maximum seven mates), pair-bonds lasted longer later in life (50% of first pair-bonds, but only 33% of all third pair-bonds, lasted one year), in both sexes, 74% retained their mate of previous year, 10% took new mate as previous mate had died and 16% divorced a living mate (with divorce more likely if pair failed to produce young in preceding season) and birds whose pair-bonds lasted > 3 years had greater reproductive success, not only due to increasing breeding experience and mate familiarity, but also initially (23). Philopatry comparatively low, with study in Bass Strait (Australia) revealing that approximately 50% of birds nest at colonies other than there natal site, while 10–14% of birds of breeding age absent from colony in any given year and another 13–18% are present but do not lay eggs (24, 25). Markedly colonial and strictly nocturnal (3), defending area around burrow entrance (20); nests in burrows , with up to 2·4 burrows/m²; burrow length averages 83 cm (3) (up to 185 cm) and is sparsely lined (20). Single white egg , mean size 72·3 mm × 47·9 mm, mass 89 g (3); incubation 52–55 days, with five stints of 10–14 (mean 12) days (3), with males typically taking marginally greater share of such duties (26); chicks have dark grey down, paler on underparts; brooded for 2–3 days then fed 0·87 meals/day at 10–19 days, but dropping to 0·258 meals/day thereafter, when adults mix short (1–3 day, 200 km) and longer (7–30 day, 1000–4000 km) trips (reaching S of Antarctic Polar Front), delivering mean meals of 78 g and 174 g, respectively (27, 28, 19); fledging c. 94 days (88–108) at mean weight of 616 g, but reaches peak mass of 860 g when 50–75 days old (3). Alternation between foraging trips of short and long duration varies significantly from year to year; in two years when sea-surface temperatures of feeding grounds used during short trips were cooler, adults initially fed their chick more frequently, then feeding decreased through chick-rearing period; in following year of warmer sea-surface temperatures, number of feedings per day was initially low but increased through chick rearing (19). Proportion of eggs that produce fledged young increases with increasing experience, from an initial 0·4–0·45 to a maximum of 0·75–0·8, before falling to 0·55–0·65 in most experienced birds (24), while probability of breeding success depends both upon breeding ages of partners and length of time they have bred together (especially the latter), and in first year of pair-bond, breeding age of female has much stronger effect than that of male in determining eproductive success (29). Overall breeding success usually 60–70% (3). Natural predators of eggs include blotched blue-tongue lizards (Tiliqua nigrolutea) and water-rats (Hydromys chrysogaster) (30), while unusually heavy summer rainfall can be a significant cause of nesting failure (burrows flooding etc.) (31). Sexual maturity at 4–6 years in males, and 5–7 years in females, athough birds start to return to colony at two years, increasing in subsequent years, with survival from fledging to first breeding estimated at 30–38% from Fisher I (Australia) study (32). Annual adult survival c. 92% for first ten years after age of first breeding, thereafter declining down to 80% for oldest birds (3). Known to have lived to at least 30 years old, with some breeding for at least 25 years (3).
Conservation Status
Not globally threatened (Least Concern). Abundant and widespread, potentially one of the most numerous of all tubenoses, with total population estimated at 13,100,000–16,500,000 pairs (33) and at least c. 30,000,000 birds at over 160 known breeding stations, especially of Victoria and Tasmania, with smaller numbers off Western Australia, South Australia and New South Wales (3); population of Tasmania may number 5,600,000 pairs in 209 colonies (but an estimated 11,500,000 burrows), with the largest on Babel I in Bass Strait with 2,860,000 burrows, and five colonies hold 52% of all burrows 70% of Tasmanian colonies are reserved from the harvesting of chicks (34). Subject to commercial exploitation for food, down, oil and fat, with formerly over 600,000 chicks harvested annually (see Family Text ); commercial activity now regulated but continues to flourish, with more recent totals of c. 250,000 chicks per annum being taken (3); trampling of burrows causes significant losses, while introduced grasses and weeds may eventually cause substantial erosion and make existing sites untenable (20). Predation by introduced mammals, especially feral cats (Felis catus) and red foxes (Vulpes vulpes) (35), responsible for some local extinctions, perhaps in combination with intense human pressure. Also, in 1980s suffered substantial mortality in N Pacific, especially off Japan, with c. 40,000 year entangled in gill nets set for salmon (36), though more recent mass-mortality incidents in same oceanic region (e.g. in 1997, hundreds of thousands starved to death in Bering Sea) (37) appear to have been caused by ecosystem changes, potentially the result of climate change (3), as well as being taken as bycatch by N Pacific driftnet fisheries, which are estimated to have accounted for 4,600,000 and 21,200,000 birds between 1952 and 2001 (38). Large ‘wreck’ of this species was recorded on Australian beaches in Oct/Nov 2013 numbering a minimum of 56,000 birds (39). Recent study indicates that both young and adults are ingesting plastic debris at sea, with potentially lethal effects (40). Some evidence that in N Pacific this species is outcompeted for food by pink salmon (Oncorhynchus gorbuscha) (41). Artificial lights attracting fledglings may cause some mortality, e.g. at Phillip Island, Australia, where 8,871 fledglings found grounded in evening and morning rescue patrols conducted during a 15-year period (1999–2013), 39% of which dead or dying; more birds were found in peak fledging, moonless and windy nights; turning the road lights off decreased the number of grounded birds (42). Maintenance of present numbers or recovery of huge former levels only possible with reduction of useless mortality; this demands elimination of introduced predators from breeding islands, and further regulation of fishing equipment to prevent drowning of birds.
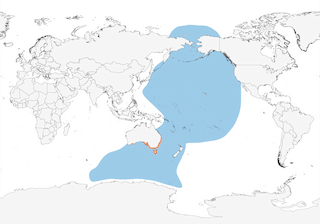
- Year-round
- Migration
- Breeding
- Non-Breeding






























