Spoon-billed Sandpiper Calidris pygmaea Scientific name definitions
- CR Critically Endangered
- Names (32)
- Monotypic
Text last updated March 8, 2017
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Лъжичкоклюн брегобегач |
| Catalan | territ becplaner |
| Chinese | 琵嘴鷸 |
| Chinese (Hong Kong SAR China) | 勺嘴鷸 |
| Chinese (SIM) | 勺嘴鹬 |
| Czech | jespák lžícozobý |
| Danish | Skeryle |
| Dutch | Lepelbekstrandloper |
| English | Spoon-billed Sandpiper |
| English (United States) | Spoon-billed Sandpiper |
| Finnish | lusikkasirri |
| French | Bécasseau spatule |
| French (France) | Bécasseau spatule |
| German | Löffelstrandläufer |
| Hebrew | חופית כפנית |
| Hungarian | Kanálcsűrű parfutó |
| Icelandic | Skeiðtíta |
| Indonesian | Kedidi paruh-sendok |
| Japanese | ヘラシギ |
| Korean | 넓적부리도요 |
| Norwegian | skjesnipe |
| Polish | biegus łyżkodzioby |
| Russian | Лопатень |
| Serbian | Sprutka kašikara |
| Slovak | pobrežník lopatkozobý |
| Slovenian | Žličasti prodnik |
| Spanish | Correlimos Cuchareta |
| Spanish (Spain) | Correlimos cuchareta |
| Swedish | skedsnäppa |
| Thai | นกชายเลนปากช้อน |
| Turkish | Kaşık Gagalı Kumkuşu |
| Ukrainian | Лопатень |
Calidris pygmaea (Linnaeus, 1758)
Definitions
- CALIDRIS
- calidris
- pygmaea / pygmaeum / pygmaeus / pygmea / pygmeum / pygmeus
The Key to Scientific Names
Legend Overview
Field Identification
14–16 cm; male averages 29·5 g, one female 34 g; wingspan 32–38 cm. Small wader closely resembling other Calidris, but has spatulate bill ; in summer, red-brown head, neck and breast with dark brown streaks; upperparts blackish with buff and pale rufous fringes . Resembles C. ruficollis, but note very different bill . Female averages slightly larger. Non-breeding adult lacks reddish coloration, but has pale brownish-grey upperparts with whitish fringes on wing-coverts; supercilium and underparts white. Juvenile has blackish upperparts with white and buff fringes; wing-coverts paler; underparts white with buff on breast; crown and broad eyestripe brown, supercilium white.
Systematics History
Subspecies
Distribution
Chukotskiy Peninsula S to N Kamchatka. Wintering area not well known, but main wintering grounds are in Bangladesh, Myanmar and Thailand; recorded in winter also locally from SE India and Sri Lanka to Indochina and S China, and S to Singapore and Philippines.
Habitat
Nests on sea coasts with sparsely vegetated sandy ridges near lakes and marshes, often near a river mouth, lagoon or sandy spit; also stream-deposited gravel strips alternating with marshy sections on dry and gravelly tundra . During non-breeding season, on muddy coasts, saltpans and mudflats in coastal lagoons, with almost no vegetation, preferring sandy shores in Japan (2). Recent late winter surveys in Bangladesh reported that foraging birds display a marked preference for firm sandy intertidal mudflats with a thin layer of soft mud collecting in ripples, spending 98% of their time feeding within small pools left by the receding tide (3).
Movement
Migratory. Moves along coasts of E Asia. During non-breeding season occurs at scattered localities on coasts of E, SE & SC Asia. Migrates N through E Russia from late May to early Jun; after breeding, adults migrate Jul to late Aug, females first (second to last week of Jul; males third week of Jul to mid Aug), and juveniles early Aug to mid Oct. Most frequently used stopover areas are S Sakhalin and W Kamchatka. Occurs on passage in North and South Korea, Japan, Taiwan, Hong Kong and E China , chiefly in late Apr to May and Sept; small numbers recorded wintering in Vietnam, Thailand , Peninsular Malaysia, Singapore, Myanmar, Bangladesh, E India (up to 14 in Sundarbans Tiger Reserve, West Bengal, Dec 2001) (4) and Sri Lanka (just three records, one of them in summer) (2, 5), with one recent (Jan 2014) record from Cambodia (6); large numbers observed only in Bangladesh, where present Aug–May, mainly Oct–Apr (up to 257 in 1989, generally many fewer since) (7, 3, 8) and in Myanmar; distribution models based on field surveys from 2005 and 2013 show key concentrations in Myanmar (main site was the upper part of the Gulf of Mottama, where the total population estimate in 2010 was 160-220) and Bangladesh, but also regular sites in China, Vietnam and Thailand (9). Vagrant to Alaska (Jun 1977, May/Jun 1986, Aug 1989, Jun 1993) and Canada (British Columbia, Jul/Aug 1978) (10). Record from the Philippines (Luzon, May 1996) not accepted (11) and same is true for claimed occurrences on Maldives (12). Some wintering areas probably still undiscovered.
Diet and Foraging
Terrestrial insects, including beetles, Hymenoptera and Diptera, small seeds, larvae of beetles and small aquatic amphipods; in winter, larval crustaceans, juvenile polychaetes (Capitellidae) and juvenile molluscs all thought to be some importance (2). Diet of chicks estimated to comprise mainly flies (Diptera, 60%), as well as Trichoptera, Hymenoptera and Carabidae beetles (35%), and seeds of plants (c. 5%) (2). On breeding grounds usually feeds around freshwater pools. Feeds in shallow water and soft, wet mud by moving head to both sides ; also picks at food items from water and mud surface; drill-like action with bill tip in mud, bill held slightly open; also observed foot-trembling. Feeds alone or in small, rather dispersed flocks and often associates with other small calidrines, e.g. C. ruficollis and C. alba, but also Tringa nebularia and Lesser Sand Plovers (Charadrius mongolus) (13), as well as C. alpina and Kentish Plovers (Charadrius alexandrinus) (2).
Sounds and Vocal Behavior
On breeding grounds gives a series of short, insect-like shrill buzzes, rendered “zhree-zhree-zhree...”. A shrill, ascending “whoeatt...whoeatt” reported in winter, and in flight gives a short “puree”, “preep” or shrill “wheet”.
Breeding
Nests Jun–Jul. Monogamous, usually for more than one season; parents feed in territory, but also in communal feeding areas. Site faithful, with c. 66% of adults returning each year, and males establishing territories (initially c. 6 ha, subsequently smaller) in same places (2). Two nests found in area of mosses, sedges and creeping osiers; one lined with dwarf willow leaves. Single-brooded, but females will lay replacement clutch if first is lost (14). Clutch four eggs, usually three in replacement clutches; incubation probably 19–23 days (2), by both adults; downy chick is warm brown mottled darker and spotted white, with largely grey-white underparts (15); chicks initially tended by both parents, but female abandons young when they are 4·5–6 days old, or immediately after hatching in late broods; males leave when chicks are 15–20 days old. Adults aggressive towards conspecifics. Breeds successfully only when lemmings (Dicrostonyx groenlandicus) present in moderate or high numbers, as predators alternatively feed on eggs and chicks when lemmings not available, e.g. at one site in 2004 eggs in c. 50% of nests hatched and approximately 60% of young that hatched successfully fledged, with 1·65 young hatching/pair and 0·8 young fledging/pair (15), but elsewhere overall breeding success has been estimated at just 7–23% (2). Predators include Arctic foxes (Alopex lagopus) (2), skuas (Stercorarius spp.), stoats and ground squirrels (14). Males do not breed before two years of age. Anual adult survival estimated at 76%, but survival of young birds is currently extremely low and seems to be one of the major drivers of the present decline (14). Oldest ringed bird more at least 16 years (16).
Conservation Status
CRITICALLY ENDANGERED. One of the most charismatic of the world’s endangered bird species, it has attracted considerable attention since its plight became apparent (17). Very small population that is declining extremely rapidly, the realisation of which (17) caused its uplisting from Vulnerable to Endangered in 2004, and to Critically Endangered in 2008 BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. . Probably always been fairly scarce due to its small range, restricted to a narrow coastal zone, combined with very specialized breeding habitat requirements, and although it has a relatively large wintering range, it occurs regularly at just a few sites BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. , the most important of which are currently estimated to be the Gulf of Mottama (Martaban, a proposed Ramsar site) and Nan Thar, Myanmar, and Sonadia I, Bangladesh (18), although there is also evidence that a significant proportion of the remaining population is wintering in SW Guangdong (S China) (19). All of the available evidence indicates that the breeding range has contracted in recent decades (18). Global population estimated at 2000–2800 pairs in 1970s, falling to 1000 pairs in 2000 (17), only 120–200 pairs by 2009 (20) and even just 40 known territories in 2011 (21), with, for example, numbers at Meinypilgyno, Chukotka, which is the most consistently monitored breeding site and holds more individuals than any other known areas, declining from c. 60 territories in 2003 to just 12 displaying males in 2009, then nine in 2012 (22) and 10–11 pairs in 2013 (18). The largest numbers recorded in recent years are 103 and 140 seen in Jiangsu province, E China, in autumn 2011 and spring 2013, respectively (23, 24). A rigorous estimate in 2014 put the global population at 210–228 breeding pairs, with a post-breeding population of adults and immatures of 661–718 individuals (25); the total winter population, however, was put at only 242–378 individuals after comprehensive field surveys from 2005 to 2013 (9). These data indicate a population reduction of 88% between 2002 and 2009, equating to an annual rate of decline of 26% (20). Declines have occurred at all known breeding sites and it is unlikely that significant colonies remain to be discovered BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. . The main threat appears to be habitat loss along the migration route (especially Korea and China) and in wintering areas (26, 20) BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. , where tidal flats are being reclaimed for industry, infrastructure and aquaculture, and are becoming increasingly polluted, while other potentially threatening habitat changes have been reported at sites in E India (27) and Bangladesh (3). Species is regularly caught for food in nets set in the key wintering areas of Bangladesh (3) and Myanmar BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. , while this issue is also known to be a threat in China (28) and Vietnam (29). Other threats on breeding grounds include hunting and collection of birds by specimen collectors (20). A consortium of organisations (in Europe and Asia) is now involved in research and conservation activities, especially in Russia, where main breeding areas are closely monitored each breeding season and many birds individually marked, to permit identification on passage and in winter without retrapping (15). There is some evidence that work to eliminate, or at least reduce, trapping of shorebirds, among them this species, on the wintering grounds is starting to have effect (30), with considerable efforts being made to raise the awareness of local peoples to its plight (18). A captive-rearing and breeding programme commenced in 2011, with eggs collected in Chukotka and young reared at Wildfowl and Wetlands Trust facilities in the UK BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. . In Aug 2013 the captive population numbered 28 birds BirdLife International (2015) Species factsheet: Calidris pygmaea. Downloaded from http://www.birdlife.org on 06/01/2015. , although to date the birds have not bred in their purpose-built aviaries (18). In addition, since 2012, eggs are being hatched and young raised in captivity close to the breeding sites, and then released to migrate S with wild-bred juveniles (31, 14, 32).
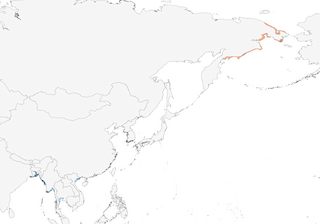
- Year-round
- Migration
- Breeding
- Non-Breeding

































