Variable Antshrike Thamnophilus caerulescens Scientific name definitions
Text last updated December 9, 2012
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | batarà pissarrós variable |
| Dutch | Grijskapmierklauwier |
| English | Variable Antshrike |
| English (United States) | Variable Antshrike |
| French | Batara bleuâtre |
| French (France) | Batara bleuâtre |
| German | Wechselameisenwürger |
| Japanese | カワリアリモズ |
| Norwegian | krattmaurvarsler |
| Polish | chronka zmienna |
| Portuguese (Brazil) | choca-da-mata |
| Portuguese (Portugal) | Choca-da-mata |
| Russian | Изменчивый колючник |
| Serbian | Raznobojni mravlji svračak |
| Slovak | batara menlivá |
| Spanish | Batará Variable |
| Spanish (Argentina) | Choca Corona Negruzca |
| Spanish (Paraguay) | Batará plomizo |
| Spanish (Peru) | Batará Variable |
| Spanish (Spain) | Batará variable |
| Spanish (Uruguay) | Batará Plomizo |
| Swedish | buskmyrtörnskata |
| Turkish | Değişken Karıncaavcısı |
| Ukrainian | Сорокуш сірий |
Thamnophilus caerulescens Vieillot, 1816
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
The Key to Scientific Names
Legend Overview
Introduction
The Variable Antshrike is an inhabitant of tropical evergreen forest edge from Peru south along the Andes to Argentina and in Eastern Brazil. As the name implies, the Variable Antshrike has an incredible variability in plumage among the eight subspecies scattered across its range. The male from the nominate race is grey overall with a black forehead and crown, black lower back and wing coverts spotted white and a pale grey belly. Females are clay colored overall with cinnamon-tawny underparts, dark brown wings and grey throat and upperbreast. Variable Antshrikes are usually encountered in the forest understory 1 to 8m from the ground, often in more open situations than other antshrikes. When foraging Variable Antshrikes make short hops, changing directions frequently and stopping every couple of seconds to scan for insects and fruit. When an insect is spotted, Variable Antshrikes lunge to glean prey from surrounding foliage or make quick upward stabs to take prey from the bottom sides of leaves.
Field Identification
14–16 cm; 15–24 g. Male nominate race has forehead and crown black , side of head grey, upperparts dark grey, mixed with black on lower back, white interscapular patch; outer scapulars edged white, wing-coverts black spotted white, flight-feathers blackish-brown, edged pale brown and white; tail brownish-black, tips white; underparts grey, belly paler, often with suggestion of scalloping. Female has crown and upperparts olive-brown with clay-coloured tinge, interscapular patch weak or absent, uppertail coverts edged yellow-brown, wing-coverts very dark brown, edged white on tips, wings dark brown with clay-coloured edgings, tail dark brown with small white tips; throat and upper breast ochraceous-grey, rest of underparts cinnamon-tawny, breast and sides tinged olive. Subadult resembles female but more buffy. Race <em>melanochrous</em> is distinctive, mostly black, variably mixed with grey on rump and lower underparts, white wing edges mostly confined to primaries, tail spots small, female with black crown becoming olive-grey on forehead and nape, wing-coverts spots very small, lower underparts cinnamon-tawny; aspersiventer differs from previous in having lower underparts scalloped black and white, tail spots larger, female with crown dark olive-grey with blackish spots; <em>dinellii</em> is paler than nominate, flight-feathers yellow-brown, underparts vary in darkness anteriorly and extent of cinnamon posteriorly, some in N part of range (“connectens”) having blackish feathers on throat and breast, no cinnamon on belly, female with crown and upperparts ochraceous grey, throat and upper breast light grey with faint yellow tinge, pale cinnamon posteriorly (approaches previous in coloration in N of range); paraguayensis is pale, lower underparts entirely white or tinged buff, female with crown olive-grey (blackish spots in W), wing-coverts olive-grey with blackish subapical spots (in E) and white tips, white below with variable yellowish-brown tinge; gilvigaster has flight-feathers edged clay colour, grey of throat and breast extending to upper belly, lower underparts washed cinnamon-tawny, female with throat to upper belly yellowish-grey, lower underparts cinnamon-tawny; ochraceiventer has upperparts and flight-feathers tinged clay colour, throat grey, remaining underparts greyish ochraceous, female with black crown mixed with olive-grey on forehead; <em>cearensis</em> has less black on crown and upperparts, female with crown rufous, wing-coverts ochraceous olive-brown (edges brightest), tail tinged clay colour, throat pale ochraceous.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Closest to T. unicolor, these two species, with T. aethiops and T. aroyae, forming a well-supported clade (1); previously considered close to T. amazonicus, but such a relationship now appears unfounded. Although complex plumage variation and unusual geographical distribution suggest present species may merit taxonomic splitting, an innovative study of vocal characters (2) found that variation in pace (notes per second) of male loudsongs was clinal and, moreover, that geographical pattern of clines accorded with genetic variation found in molecular study (3). Other proposed races seem indistinguishable or plumage differences appear clinal: thus, subandinus (N Peru) synonymized with melanochrous; connectens (EC Bolivia) with dinellii; albonotatus (EC Brazil) with nominate; and pernambucensis (NE Brazil) with cearensis. Further study required to examine if there is any parapatry without intergradation among accepted races. Eight subspecies recognized.Subspecies
Thamnophilus caerulescens melanochrous Scientific name definitions
Distribution
Thamnophilus caerulescens melanochrous Sclater & Salvin, 1876
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- melanochroa / melanochrous
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens aspersiventer Scientific name definitions
Distribution
Thamnophilus caerulescens aspersiventer d'Orbigny & de Lafresnaye, 1837
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- aspersiventer / aspersiventris
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens dinellii Scientific name definitions
Distribution
Thamnophilus caerulescens dinellii Berlepsch, 1906
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- dinelliana / dinellianus / dinellii
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens paraguayensis Scientific name definitions
Distribution
Thamnophilus caerulescens paraguayensis Hellmayr, 1904
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- paraguayae / paraguayensis
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens gilvigaster Scientific name definitions
Distribution
Thamnophilus caerulescens gilvigaster Pelzeln, 1868
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- gilvigaster
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens caerulescens Scientific name definitions
Distribution
Thamnophilus caerulescens caerulescens Vieillot, 1816
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens ochraceiventer Scientific name definitions
Distribution
Thamnophilus caerulescens ochraceiventer Snethlage, 1928
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- ochraceiventer / ochraceiventris
The Key to Scientific Names
Legend Overview
Thamnophilus caerulescens cearensis Scientific name definitions
Distribution
Thamnophilus caerulescens cearensis (Cory, 1919)
Definitions
- THAMNOPHILUS
- thamnophilus
- caerulescens
- cearae / cearensis
The Key to Scientific Names
Legend Overview
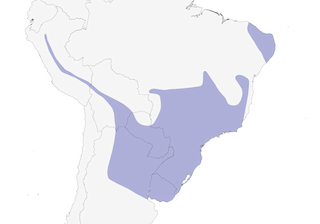
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Understorey and mid-storey of evergreen forest, second-growth woodland, and patches of thickets and trees in open regions. More a bird of forest edge, open viny woodland and dense thickets than of mature forest interior, and often persists in highly degraded remnant patches. In N part of Andean range (melanochrous, aspersiventer) inhabits thickets and undergrowth of humid forest, also dense stunted forest on low-elevation ridge crests, at 1200–2800 m; farther S in Andes and at lower elevations in NW Argentina (dinellii), found most often in dense undergrowth under patches of trees in woodlands of foothills and plains, especially in riparian scrubland and shrubby hillsides in canyons, at 200–2700 m. In the Chaco of Bolivia and Paraguay (paraguayensis) occupies patches of tall dense shrubs and low trees in riparian situations, as well as semi-deciduous scrub-forest, at 150–850 m. In NE Argentina, Uruguay and extreme SE Brazil (gilvigaster) restricted to thickest woods of all types, which most often found along banks of streams and in swampy areas, to 1050 m; farther N (caerulescens), especially common in vine-tangled zones of steep hillsides and also occurs in bamboo, vine tangles on streamside banks, dense second growth and, locally, restinga (low, dense, stunted forest off sandy beaches), to 1300 m. In the Planalto (ochraceiventer), inhabits gallery forest and small woodlots of the highlands, at 750–1350 m. In NE Brazil (cearensis) restricted to remnant patches of humid or semi-humid forest with abundant vines, to 850 m, and absent from immediately adjacent caatinga or dry-forest habitats.
Movement
Ringing at a site in N Argentina (Salta) suggests some movement in S; presumed resident throughout remainder of range.
Diet and Foraging
Little published. Feeds primarily on insects, including moths and larval lepidopterans, katydids (Tettigoniidae), grasshoppers (Acrididae), mantids (Mantidae), stick-insects (Phasmatidae), true bugs (Hemiptera, Homoptera) and beetles (Coleoptera), as well as spiders and other arthropods; also seeds. A pair in Brazil (Rio de Janeiro) seen to consume three melastome (Melastoma) fruits; the birds severed the fruit at the base, swallowed it, then spat out what appeared to be a papery skin or husk. Also observed feeding on berries of mistletoe (Myrsinaceae). Forages in pairs or singly, 1–8 m above ground (mostly 1–5 m), often in more open situations than other antshrikes, but also working dense crowns of bamboo where these form interlacing mats of vegetation with crowns of understorey trees. Great individual variation in pace of foraging; often progresses deliberately by short hops separated by pauses of 3–15 seconds (typical of most antshrikes), but more frequently moves steadily and somewhat rapidly (pauses seldom more than 1–2 seconds) with series of small hops (none more than a few centimetres), looking about constantly and changing direction more frequently than most congeners; regardless of pace, seems to spend long periods of time thoroughly covering small areas. Reaches out, up or down, or lunges to glean prey from all leaf, stem, vine and branch surfaces, using quick bill-stabbing motions; also regularly makes short (15–40 cm) jumping horizontal or downward strikes to pluck items from tops of leaves, and vertical sallies of up to 1 m to hover-glean from underside of overhanging vegetation. Often creeps up lianas and investigates them for food. Occasionally forages less than 1 m from ground, usually clinging laterally to slender vertical stems and then pouncing on arthropods in leaf litter, before jumping back up to a perch to scan. Sometimes hops about slowly on ground, peering around. While foraging, habitually flicks wings and dips the tail slowly before rapidly flicking it upwards (applies to nominate, dinellii, gilvigaster and cearensis; no data on other races). Often joins mixed-species flocks, but just as frequently alone. Nominate race occasionally reported as following army ants (both Eciton burchelli and Labidus praedator).
Sounds and Vocal Behavior
Loudsong a simple countable series of usually 6–7 plaintive, evenly spaced whistles of same pitch and intensity (rarely, introductory or final notes differ slightly in pitch or intensity), note shapes and pace variable, perhaps regionally. Call a single note, usually short and nasal, typically repeated in long sequences but at highly variable pace; also longer, softer note and raspy growl.
Breeding
Oct–Feb in Brazil, Aug–Nov in Bolivia and Peru and Oct–Dec in Argentina. Nest of dinellii a well-woven cup, diameter 6 cm, depth 5 cm, made of fine grass leaves, stems and stalks, lined with thinnest stalks and horsehairs or similar fine rhizomorphs, suspended by rim (connecting points additionally bound by spider webs) from thin branches of horizontal fork 0·6–2·5 m above ground in bush; nests in Argentina (gilvigaster and nominate race) similar; one nest of nominate race in E Brazil (Espírito Santo) a pendent cup 8 cm high, 10 cm across, covered on outside with moss, some hanging 7·5 cm below nest, placed 1·8 m up in a Tibouchina bush at edge of road in tall second growth; in NE Brazil (cearensis) a deep purse-shaped cup, loosely woven (contents visible from below) with coarser grasses and plant fibres, lined with fine black, horsehair-like rhizomorphs, frequently green leaves or moss woven into exterior, suspended by rim from thin horizontal fork (fibres of nest looped over branches at three obvious points of attachment) among foliage in understorey sapling or broad-leaf shrub, often with overhanging large leaf a few centimetres above rim, 6 nests 0·7–7·5 m above ground. Clutch size known for nominate race, cearensis, dinellii and gilvigaster, normally three eggs, less often two, creamy white with irregular (and individually variable) purplish-brown blotches, speckles, occasional streaks, sometimes also ash-grey spots (Argentina), markings mostly concentrated around larger end; incubation by both sexes during day, both also feed chicks, no information on incubation and nestling periods; Espírito Santo nest (nominate) held an apparently infertile egg and a nestling, egg pinkish-white with large red-brown spots on larger end, a few elsewhere, male gradually left care of single nestling to the female. Nest sometimes parasitized by Shiny Cowbird (Molothrus bonariensis) in Argentina.
Conservation Status
Not globally threatened. Fairly common over most of its extensive range, which includes a number of protected areas. This species’ adaptation to edge habitats, second-growth woodland and other human-created habitats makes it less sensitive to disturbance. In Brazil, in a study of remnant woodland patches in Paraná which ranged in size down to 0·5 ha, this was the only antbird to survive in all 13 patches; similarly, it was the only thamnophilid among the most frequently observed bird species surviving in a eucalyptus (Eucalyptus) plantation in Minas Gerais. In NE Brazil, however, the conservation of race cearensis is of concern, since humid forest within its range has been largely reduced to remnant patches (e.g. Murici Ecological Reserve and Pedra Talhada State Park, in Alagoas) surrounded by sugar cane and other large-scale agricultural development. One stronghold of this race is the Serra de Baturité region, which has been categorized as a Secondary EBA (Ceará Caatinga and Serras) and is home to two restricted-range species, the Ochraceous Piculet (Picumnus limae) and the Buff-breasted Tody-tyrant (Hemitriccus mirandae), that are classified as Vulnerable. The few remaining areas of humid and semi-deciduous forest habitats in the Baturité region are still being cleared, and are in need of protection.
- Year-round
- Migration
- Breeding
- Non-Breeding















































