Mandarin Duck Aix galericulata Scientific name definitions
- LC Least Concern
- Names (46)
- Monotypic
Text last updated May 7, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Mandaryneend |
| Albanian | Rosa mandarinë |
| Arabic | بط صيني |
| Asturian | Corñu mandarñn |
| Basque | Mandarin-ahatea |
| Bulgarian | Мандаринка |
| Catalan | ànec mandarí |
| Chinese | 鴛鴦 |
| Chinese (Hong Kong SAR China) | 鴛鴦 |
| Chinese (SIM) | 鸳鸯 |
| Croatian | mandarinka |
| Czech | kachnička mandarínská |
| Danish | Mandarinand |
| Dutch | Mandarijneend |
| English | Mandarin Duck |
| English (United States) | Mandarin Duck |
| Faroese | Seglont |
| Finnish | mandariinisorsa |
| French | Canard mandarin |
| French (France) | Canard mandarin |
| Galician | Pato mandarín |
| German | Mandarinente |
| Greek | Μανδαρίνος |
| Hebrew | מנדרין סיני |
| Icelandic | Mandarínönd |
| Italian | Anatra mandarina |
| Japanese | オシドリ |
| Korean | 원앙 |
| Latvian | Mandarīnpīle |
| Lithuanian | Mandarininė antis |
| Mongolian | Мандир уранхажин |
| Norwegian | mandarinand |
| Polish | mandarynka |
| Portuguese (Portugal) | Pato-mandarim |
| Romanian | Rață mandarin |
| Russian | Мандаринка |
| Serbian | Mandarinka |
| Slovak | kačička mandarínska |
| Slovenian | Mandarinka |
| Spanish | Pato Mandarín |
| Spanish (Ecuador) | Pato Mandarín |
| Spanish (Spain) | Pato mandarín |
| Swedish | mandarinand |
| Thai | เป็ดแมนดาริน |
| Turkish | Mandarin Ördeği |
| Ukrainian | Мандаринка |
Aix galericulata (Linnaeus, 1758)
Definitions
- AIX
- aix
- galericulata / galericulatus
The Key to Scientific Names
Legend Overview
Field Identification
41–51 cm; male 571–693 g, female 428–608 g (1); wingspan 68–74 cm (2). Male has eclipse plumage , but in breeding plumage is completely unmistakable due to orange wing sails and side whiskers: forehead and crown glossy green to purplish, becoming coppery red and white on long nuchal crest, buff cheeks with chestnut ruff on neck-sides and foreneck, mantle and back olive-brown, scapulars glossy blue marked white and black, tertials blue, with orange-buff outer webs, upper breast maroon, lower breast to undertail-coverts white, with black and orange-white bars on flanks, tail brown, glossed green, with long dark blue-green uppertail-coverts; bill red with flesh-pink nail, legs and feet orange-buff and eyes dark brown with yellowish outer ring. Female (and eclipse male) resemble same plumages of A. sponsa but narrower white ring around eye typically extends as line behind it, have different bill pattern (greyish brown with pale orange tip), pale grey head with finely streaked ear-coverts, more heavily spotted underparts, striking white lines on inner secondaries, less clear-cut white throat and paler legs (3), although there may be some variation in these features; also have different bill shape. Juvenile resembles female, though female has pinkish bill, and young are more uniformly grey-brown with less distinct face markings and less obviously dappled appearance to upper breast and flanks.
Systematics History
Subspecies
Introduced in Britain and several countries of C Europe, also in USA.
Hybridization
Hybrid Records and Media Contributed to eBird
-
Wood x Mandarin Duck (hybrid) Aix sponsa x galericulata
Distribution
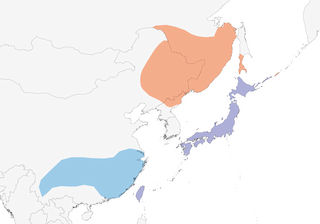
Scattered in Russian Ussuriland, Sakhalin I, NE China, Korea, Japan. Continental populations winter in S China.
Habitat
Pools, lakes , rivers , marshes and swamps surrounded by dense deciduous forest. Has a preference for small islands and waterbodies with abundant emergent vegetation and wooded islets. Generally found below 1500 m (2), but recorded to at least 2700 m (Ailao Mts, Yunnan) (4).
Movement
Asian population essentially migratory, wintering at lower latitudes in E, C & S China, as well as Korea (where now known also to breed) (5), Taiwan (which also has resident population) (6) and Honshu (Japan) (1), arriving in Ussuriland and on Sakhalin in Mar to early Apr (1). Migrants pass through Beidahe (Hebei, NE China) between 9 Sept and 2 Nov in autumn, and 9 Apr to 3 May in spring (7). However, Japanese and British feral birds mostly sedentary. Recent radio-tracking of resident population on Taiwan, involving 35 males (29 adults) and 46 females (41 adults), found that home ranges of both sexes were smaller during the breeding season than outside it (15·2 ± 7·9 km in males versus 16·8 ± 7·3 km), while those of males (8·8 ± 5·9 km) were significantly larger than home ranges of females (4·3 ± 3·2 km) during the breeding season; young females appear to show stronger philopatry than young males, with the longest-recorded movement by any bird being 35 km (8). Wild (or presumed) birds have occurred in NE India (Assam Valley, 1901/02; Uttarakhand, Feb 1999; and Manipur, Mar 1934, 1997 and Dec 2013) (9, 10), E Nepal (Jan 1990) (11), NE Bangladesh (Jan 1993) (12), Bhutan (Mar 2002) (13), Burma (Dec 1933), Hong Kong (six records 1958–1984), Hainan, China (Dec 2003) (14), Laos (Jan 2004, Jan 2005) (15), Philippines (Luzon, Nov–Dec 2013) (16), Vietnam and N Thailand (most records recent) (17, 16), and escapes throughout Europe (where records available from Azores (18), Iceland, Faeroes, Sweden, Finland, Poland, Czech Republic, Spain (where perhaps breeds in Valencia) (19, 20, 21), Balearic Is, Canaries (20, 3), Italy, Bulgaria, Romania, Belarus and Morocco) (2) and North America (California) (18).
Diet and Foraging
Seeds, particularly nuts, acorns, sweet chestnut, beechmast (1) and grain, aquatic plants and animal food (land snails, frogs, tadpoles (1), insects, fish). Invertebrates particularly important in diet of ducklings (1). Regularly feeds in flooded rice fields after harvest in Oct (1), but such foraging behaviour is said to occur year-round in Japan and Korea (22). Feeds both by day (most active early morning and evening) (1) and at night by dabbling on water surface and shoreline (1), and by head dipping and upending in shallow waters; rarely dives (1).
Sounds and Vocal Behavior
Generally rather quiet, usually vocalizing only when disturbed or in courtship: male calls mainly variations on sharp, rising whistle with a lower, snorting, nasal component, rendered “hwick” or “uib uib” (1), whereas principal call of female is a loud, sharp, single-noted “kett” or “ke”, which might easily be mistaken for a Common Coot (Fulica atra), if bird not seen, as well as a repeated, plaintive “ack”, which can be uttered at rate of up to 53/minute in display (so-called “Coquette call”), sometimes also in flight, with a shorter version when disturbed (2).
Breeding
Starts Apr in England and Russia, but apparently not until Jun in Japan (23); in NE China egg-laying occurred 22 Apr to 11 Jun in one study (24). Monogamous but not territorial with pair-bond lasting until late in incubation period, more rarely brood-rearing (typically longer than in A. sponsa), with pair-bonds usually established on wintering grounds (from Sept, mainly Feb–Mar) (2, 1). In single pairs; nests mainly in tree hollows, selected by both pair-members, including old woodpecker holes (perhaps especially those of Black Woodpecker Dryocopus martius) (25) but also natural holes and nestboxes (1), up to 15 m above ground (usually to 10 m) (2); may return to same hole in consecutive seasons and in native range perhaps competes with Mergus merganser and M. squamatus for suitable cavities (1), which may be up to 0·5 km from rivers (26); rarely nests on ground in dense vegetation, under bush or fallen ground (2); once in a building in S China (27). In England, dependent on natural nest-holes, particularly beech (Fagus silvatica), and nestboxes (1), while competitors for nest-sites in naturalized range include Jackdaws (Corvus monedula), Mergus merganser, Tawny Owls (Strix aluco) and Bucephala clangula (28). Clutch 7–14 white or buff eggs (1) (occasionally up to 25 (24), as result of dump nesting, which is apparently common in England and NE China) (1, 24), laid at one-day intervals (exceptionally up to six) (24), size 46–55 mm × 41–43 mm, mass (in captivity) 35·5–51·5 g (1); incubation 28–33 days by female alone (1); chicks have olive-brown down above, yellowish below, on wings and rump, with grey-purple bill, mass 18–33·5 g (in captivity) when one day old, and hatch synchronously (1); fledging c. 40–45 days, with young cared for entirely by female (1). Incidence of intraspecific parasitism recently quantified in NE China, where 46·2% of completed clutches are parasitized and frequency is positively associated with nest density, but declines as the season progressed (as in A. sponsa); on average, c. 2·5 females lay eggs in each parasitized nest, with significant differences in clutch initiation dates and mean laying period between parasitized and unparasitized nests, and clutch size for unparasitized nests decreases with advancing initiation date (24). In same study, nest desertion was main cause for egg failures, accounting for 72·8%, followed by non-fertilization (11·4%) and human disturbance (11·1%); other losses were due to embryo death and unknown reasons, while five ducklings in three parasitized nests with large number broods died before leaving the nest, apparently being trampled; the hatching rate for eggs in successful nests was 87·1%, with no significant difference between parasitized and unparasitized nests, while mean numbers of ducklings leaving successful unparasitized and parasitized nests was 8·4 and 15·4, respectively (24). Data on fledging success in wild lacking, but egg predators include American mink (Mustela vison), raccoon dogs (Nyctereutes procyonoides) and martens (Martes spp.), but no information concerning their effects; in captivity especially prone to avian tuberculosis, like A. sponsa, but relatively immune to aspergillosis and candidiasis (1). Predators of adults include Siberian weasel (Mustela sibirica), Northern Goshawk (Accipiter gentilis) and Tawny Fish-owl (Ketupa flavipes) in native range (6), and largemouth bass (Micropterus salmoides) in California (18). Sexual maturity at one year (both sexes, in captivity). Adult annual survival studied on Taiwan, where rates of 74–80% for males and 44–50% for females recorded (with most of the latter lost during the physiologically demanding breeding period and the disparity apparently producing a heavily skewed sex bias towards males) (6, 8); similarly, in an introduced population in Germany annual survival was 65% in first-year males, 66% in adult males, 47% first-year females and 57% for adult females, these differences being fully explained by sex differences in survival; female mortality but not male mortality peaked during the breeding season (29). Longevity records, oldest bird in captivity was 15 years old, while a free-flying bird in England was 11 years old (1).
Conservation Status
Not globally threatened (Least Concern). At present, majority of Asian population, which was estimated at just 6100–6600 pairs in mid 1980s, occurs in Japan, with recent (early 2000s) population estimates of 20,000 in China, 5000 in Korea and 40,000 in Japan based almost entirely on winter data (1). In winter 1990 census, 13,361 birds counted in Japan , and 2332 in partial count in China (where overall wintering population is thought to 3000–4000 birds, with most on middle and lower reaches of Changjiang R, L Taihu and L Dongting) (30). Only minimally significant available figures on continental population are: 100 individuals counted during autumn/winter 1986 censuses in Taesong’dong and Panmucnch’om Marshes, South Korea, but up to 2550 were on Cheju I in Jan–Mar 1999 (31); in early 2004, some 718 were counted at sites in Jiangxi (32) with c. 1000 in Wuyuan (NE Jiangxi) subsequently (33), and flocks of 40–50 occur in early spring in Changbai Shan Nature Reserve, one of the breeding areas in NE China, where species nests mainly on upper reaches and tributaries of the Songhua, Yalu, Mundanjiang and Tumen rivers, being especially common on the Toudaobai, Erdaobai, Gutong, Tan Wang and Nancha rivers in Changbai and Xiao Xinganling, with an estimated overall population of 1000–1500 pairs in 1990s (30). In NE China, declines specifically noted on Toudaobai R in Changbai Mts, where breeding density in 1977/78 averaged 1·8 birds/km, but had declined to 0·90 birds/km by 1994 (30). In Japan was very common resident but greatly declined due to overhunting, with recovery after protection in 1947; notable increase in wintering population in particular, but not known if this because of increased breeding population or continental visitors; now considered local and uncommon, with further moderate increase in numbers wintering noted during 1996 to 2009 (34, 35). Long thought not to breed in Korea, but evidence is available from several regions of the peninsula, although there is no population estimate (5). Relatively small numbers (c. 300–500 birds) resident in C Taiwan (6). In Far East of Russia, has vanished from deforested basin of L Khanka and valley of Ussuri R, where now restricted mainly to its major tributaries (Khor, Bikin and Iman rivers); in 1990s considered to be still common on lower and middle Bikin, including its larger lower tributaries, the Alchan, Ulitka, Kushnarikha and Zmeinaya, with c. 350–400 pairs on the Bikin and c. 300 pairs in Amurland in the 1980s, with no evidence of a decline in the first-named during the 1990s, but is now largely confined to protected areas in S Primorye (26). British feral population of c. 7000 in 1988 significant; currently increasing in both numbers and range, but no up-to-date population estimate (36). Much smaller feral but often increasing populations more or less established in Ireland (2–5 pairs in early 1990s), France (5–10 pairs early 1990s, growing to 27–36 pairs and 135–140 individuals in 2011) (37), Belgium (first bred 1987, up to ten pairs subsequently, 80–95 pairs by late 1990s) (28), Netherlands (c. 50–60 pairs in 1992–1994) (28), Germany (> 50 pairs in Berlin, smaller numbers scattered elsewhere, increasing to 430–600 pairs in whole country by 2005–2009) (38), Denmark (c. 10 pairs in late 1980s), Austria (< 10 pairs in 1993) (2) and Switzerland (first bred 1958, 10–20 pairs and up to 200 birds in 1985–1997) (39, 28); also bred in Norway in 1970/71 (2). Population in California (USA) estimated at 550 birds in mid 1980s (18). Decline in continental Asia related to habitat destruction (through expansion of mining, agriculture and other industry) and exportation from China in vast numbers until as late as 1975 (1).
- Year-round
- Migration
- Breeding
- Non-Breeding































