Lesser Spotted Woodpecker Dryobates minor Scientific name definitions
Text last updated May 9, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Qukapiku larosh i vogël |
| Armenian | Փոքր փայտփոր |
| Asturian | Piquetñn comñn |
| Azerbaijani | Kiçik ağacdələn |
| Basque | Okil txikia |
| Bulgarian | Малък пъстър кълвач |
| Catalan | picot garser petit |
| Chinese (SIM) | 小斑啄木鸟 |
| Croatian | mali djetlić |
| Czech | strakapoud malý |
| Danish | Lille Flagspætte |
| Dutch | Kleine Bonte Specht |
| English | Lesser Spotted Woodpecker |
| English (United States) | Lesser Spotted Woodpecker |
| Finnish | pikkutikka |
| French | Pic épeichette |
| French (France) | Pic épeichette |
| Galician | Picapau pequeno |
| German | Kleinspecht |
| Greek | Νανοδρυοκολάπτης |
| Hebrew | נקר גמדי |
| Hungarian | Kis fakopáncs |
| Icelandic | Stubbspæta |
| Italian | Picchio rosso minore |
| Japanese | コアカゲラ |
| Latvian | Mazais dzenis |
| Lithuanian | Mažasis margasis genys |
| Mongolian | Цоохор тоншуул |
| Norwegian | dvergspett |
| Persian | دارکوب خالدارکوچک |
| Polish | dzięciołek |
| Portuguese (Portugal) | Pica-pau-galego |
| Russian | Малый пёстрый дятел |
| Serbian | Mali detlić |
| Slovak | ďateľ malý |
| Slovenian | Mali detel |
| Spanish | Pico Menor |
| Spanish (Spain) | Pico menor |
| Turkish | Küçük Ağaçkakan |
| Ukrainian | Дятел малий |
Dryobates minor (Linnaeus, 1758)
Definitions
- DRYOBATES
- dryobates
- minor
The Key to Scientific Names
Legend Overview
Field Identification
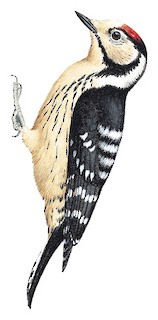
14–16 cm; 16–25 g (hortorum), 18–22 g (comminutus), 19–26 g (minor). Male has buff-tinged white forehead and lores, crimson-red crown (usually some pale feather bases showing) bordered by narrow black lines, black nape and hindneck; rest of head, including chin and throat, white or whitish, with black malar stripe expanding on neck side and extending irregularly down to upper breast side; black upperparts, broad white bars on lower mantle and lower scapulars to upper rump, wing-coverts with broad white spots or bars near tips; black flight-feathers broadly barred white; uppertail black, outer feathers white with 2 or 3 dark bars near tip; white underparts with slight buff tinge, breast sides and flanks with thin black streaks, undertail-coverts usually spotted black; short bill chisel-tipped, dark grey to blackish, paler base of lower mandible; iris red-brown or brownish; legs greenish-grey. Female lacks red on head, has white or buffish-white forecrown, black crown sides and hindcrown. Juvenile duller than adult, black areas tinged brown, pale forehead patch obscured by darker tips, heavier but duller and less defined streaks below, male with greyish-mottled pinkish-red forecrown, female with pale forecrown obscured by dark tips and usually a few reddish tips. Races differ in size, plumage darkness and pattern, but variation often clinal and many intermediates: kamtschatkensis is largest, long-winged, has proportionately longer bill than nominate, much more white above, almost unmarked white outer tail, few or no streaks below; amurensis is very like nominate, but narrower white bars above, slightly greyer and usually more streaked below; <em>hortorum</em> has slightly less white on back than nominate, outer rectrices more barred, buff or light brown face and underparts, throat sometimes tinged pink, flanks more heavily streaked; buturlini is darker than previous, more heavily streaked below; comminutus resembles previous, but slightly smaller, with much fainter streaks below; ledouci is also dark, but pale areas of head to breast somewhat darker buff-brown, often heavier streaking below, may show some black behind ear-coverts, usually has all-black bill; danfordi is similarly dark below, sometimes brown-tinged, has black band from hindcrown around rear ear-coverts; colchicus is like previous, but has more white above, black band behind ear-coverts sometimes broken; quadrifasciatus is small, short-billed, has less white above, sometimes hint of black band joining malar with nape, outer rectrices heavily barred black, pale areas buff-brown, flanks heavily dark-streaked and sometimes barred; morgani is distinctive, has very long and narrow bill, broad black band behind ear-coverts, buff face, deep buffish-brown upper breast (and sometimes chin and throat) contrasting with white rest of underparts, very sharp black streaks on breast and flanks.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Previously placed in Dendrocopos or Picoides. Recent molecular analyses found this species to be closest to D. cathpharius (with D. pernyii), these also being close to D. pubescens (1). Races intergrade extensively over species’ huge range, with many intermediate populations; numerous additional races have been described, often on rather weak grounds, and differences frequently part of a clinal variation. Other proposed races include hispaniae (Spain), jordansi (C European mountains), serbicus (Montenegro), wagneri (Romania), hyrcanus (N Iran), mongolicus (SW Siberia to Mongolia), nojidoensis (NE Korea) and immaculatus (Kamchatka). Eleven subspecies currently recognized.Subspecies
Dryobates minor comminutus Scientific name definitions
Distribution
Dryobates minor comminutus (Hartert, 1907)
Definitions
- DRYOBATES
- dryobates
- minor
- comminutus
The Key to Scientific Names
Legend Overview
Dryobates minor hortorum Scientific name definitions
Distribution
Dryobates minor hortorum (Brehm, 1831)
Definitions
- DRYOBATES
- dryobates
- minor
- hortorum
The Key to Scientific Names
Legend Overview
Dryobates minor buturlini Scientific name definitions
Distribution
Dryobates minor buturlini Hartert, 1912
Definitions
- DRYOBATES
- dryobates
- minor
- buturlini
The Key to Scientific Names
Legend Overview
Dryobates minor minor Scientific name definitions
Distribution
Dryobates minor minor (Linnaeus, 1758)
Definitions
- DRYOBATES
- dryobates
- minor
The Key to Scientific Names
Legend Overview
Dryobates minor amurensis Scientific name definitions
Distribution
Dryobates minor amurensis (Buturlin, 1908)
Definitions
- DRYOBATES
- dryobates
- minor
- amurensis
The Key to Scientific Names
Legend Overview
Dryobates minor kamtschatkensis Scientific name definitions
Distribution
Dryobates minor kamtschatkensis (Malherbe, 1860)
Definitions
- DRYOBATES
- dryobates
- minor
- kamschaticus / kamtschatica / kamtschaticus / kamtschatkensis / kamtschatschensis
The Key to Scientific Names
Legend Overview
Dryobates minor colchicus Scientific name definitions
Distribution
Dryobates minor colchicus (Buturlin, 1908)
Definitions
- DRYOBATES
- dryobates
- minor
- colchica / colchicus
The Key to Scientific Names
Legend Overview
Dryobates minor danfordi Scientific name definitions
Distribution
Dryobates minor danfordi (Hargitt, 1883)
Definitions
- DRYOBATES
- dryobates
- minor
- danfordi
The Key to Scientific Names
Legend Overview
Dryobates minor quadrifasciatus Scientific name definitions
Distribution
Dryobates minor quadrifasciatus (Radde, 1884)
Definitions
- DRYOBATES
- dryobates
- minor
- quadrifasciata / quadrifasciatus
The Key to Scientific Names
Legend Overview
Dryobates minor morgani Scientific name definitions
Distribution
Dryobates minor morgani (Zarudny & Loudon, 1904)
Definitions
- DRYOBATES
- dryobates
- minor
- morgani / morganii
The Key to Scientific Names
Legend Overview
Dryobates minor ledouci Scientific name definitions
Distribution
Dryobates minor ledouci (Malherbe, 1855)
Definitions
- DRYOBATES
- dryobates
- minor
- ledouci
The Key to Scientific Names
Legend Overview
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Temperate and boreal deciduous woodland in lowlands. Open forest with softwood deciduous trees in vicinity of lakes or rivers are preferred; also forest edge, and in parks, orchards and gardens. Requires good number of thin snags, as in old stands or in riparian woodland. Restricted to cork-oak forest, especially with Quercus suber, in N Africa. Lowlands and foothills to 850 m, locally to 1260 m, in Europe; at higher elevations in Asia, to 1400–2000 m in Caucasus, 1700 m in Altai Mts, and 1400 m in Mongolia; to 1300 m in N Africa.
Movement
Resident in most of range. N populations partly migratory; movements often eruptive in nature, in parallel with movements of D. major. Autumn migration mainly late Aug to Nov; N European birds may reach C Europe and Black Sea.
Diet and Foraging
Small insects comprise main bulk of diet. In summer, mostly caterpillars , aphids, ants, beetles, and other surface-dwelling arthropods, including Diptera and spiders; even small snails (Discus) taken. In winter, wood-boring larvae (e.g. Buprestidae, Cerambycidae, Curculionidae) and those living under the bark (e.g. Scolytidae, Ipidae) become important. Ants more important in S parts of range. Eats very little vegetable matter, but occasionally takes fruit and berries, and seeds at feeders. Generally forages singly; may join mixed flocks, especially with tits (Paridae), outside breeding season. Forages quietly in upper stratum or in dense vegetation. Prefers to seek food from (vertical) twigs 1–3 cm in diameter in the canopy, rarely on the trunk or base of large trees; descends to lower levels mainly to visit bushes and plant stalks, latter including particularly reed (Phragmites), occasionally corn. Foraging techniques include gleaning, hammering, pecking series to dislodge large pieces of bark, and probing; gleaning predominates when foraging for nestling food. Also makes aerial sallies for insects, and visits sap holes made by other woodpecker species.
Sounds and Vocal Behavior
Commonest is series of notes , “gee-geegeegee” or longer; single “gig” and various other calls rarely heard. Both sexes drum , characteristic long high-pitched rolls at 14–19 per minute, often interspersed with call series at height of courtship.
Breeding
Laying Apr to mid-May, to Jun in N. Generally monogamous, pair-bond may extend over several years; also, study in S Sweden found almost 10% of females polyandrous, c. 3% of males polygynous. Courtship from Feb, most conspicuous displays wing-spreading and tail-spreading, characteristic flutter-aerial display, and gliding flight with wings held well above back. Nest-hole excavated by both sexes, male often taking greater share, work lasting 2–4 weeks, sometimes as few as 6 days; at 0·4–20 m, usually below 8 m, in soft wood of dead or decaying trunk, stump or underside of a branch, hole entrance 3–3·5 cm, cavity depth 10–18 cm; nestbox used rarely; territory often small. Clutch 5–6 eggs, rarely 3 or up to 9; incubation by both sexes, male often doing more, period 10–12 days; chicks fed by both parents , fledging period 18–21 days, rarely up to 23 days; juveniles cared for by parents for a further 1–2 weeks. Success variable; in study in S Sweden, 34% of breeding attempts failed to produce fledglings, and polyandrous females c. 40% more successful than monogamous ones. First breeding at 1 year.
Conservation Status
Not globally threatened (Least Concern). Fairly common to scarce in most of range; nowhere very common; scarce in N Africa. European population estimated at c. 195,000–240,000 pairs, of which 40,000–60,000 in Belarus, c. 30,000–50,000 in Germany, 20,000–40,000 in Poland, 20,000–30,000 in Hungary. Densities generally low, e.g. 2–3 pairs/100 ha in primeval deciduous forest in Poland, and elsewhere in Europe only 0·06–1·6 pairs/km². Appears to have declined in many parts of Europe, largely as a result of loss of deciduous habitats, especially riverine forest and old orchards, which were previously used extensively. It is however spreading in parts of Spain, using poplar plantations (3). Responds negatively to forest fragmentation and admixture of conifers. Main conservation measure required is to ensure that adequate suitable habitat remains for this species.
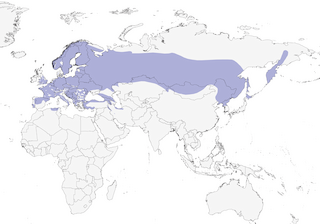
- Year-round
- Migration
- Breeding
- Non-Breeding