Siberian Rubythroat Calliope calliope Scientific name definitions
- LC Least Concern
- Names (40)
- Monotypic
Text last updated January 21, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Сибирска червеногушка |
| Catalan | rossinyol siberià |
| Chinese | 野鴝 |
| Chinese (Hong Kong SAR China) | 紅喉歌鴝 |
| Chinese (SIM) | 红喉歌鸲 |
| Croatian | crvenovoljka |
| Czech | slavík Kalliopin |
| Danish | Rubinnattergal |
| Dutch | Roodkeelnachtegaal |
| English | Siberian Rubythroat |
| English (United States) | Siberian Rubythroat |
| Faroese | Øsreyður náttargali |
| Finnish | rubiinisatakieli |
| French | Rossignol calliope |
| French (France) | Rossignol calliope |
| German | Rubinkehlchen |
| Greek | Ρουμπινολαίμης |
| Hebrew | אדום-גרון |
| Hungarian | Rubinbegy |
| Icelandic | Fagurgali |
| Italian | Calliope |
| Japanese | ノゴマ |
| Korean | 진홍가슴 |
| Latvian | Sibīrija rubīnrīklīte |
| Lithuanian | Sibirinė raudongurklė |
| Mongolian | Өнгөлүүрт гургалдай |
| Norwegian | rubinstrupe |
| Polish | słowiczek rubinowy |
| Portuguese (Portugal) | Pisco-de-garganta-vermelha |
| Romanian | Gușă roșie siberiană |
| Russian | Соловей-красношейка |
| Serbian | Crvenovoljka |
| Slovak | slávik červenohrdlý |
| Slovenian | Rubinasti slavec |
| Spanish | Ruiseñor Calíope |
| Spanish (Spain) | Ruiseñor calíope |
| Swedish | rubinnäktergal |
| Thai | นกคอทับทิม |
| Turkish | Sibirya Yakut Gerdanlı Bülbülü |
| Ukrainian | Соловейко червоногорлий |
Calliope calliope (Pallas, 1776)
Definitions
- CALLIOPE
- calliope
The Key to Scientific Names
Legend Overview
Field Identification
14–16 cm; 16–29 g. Male breeding is warm olive-brown from crown to tail, with bold white supercilium and submoustachial stripe , black lores and malar line; metallic pale ruby-red chin and throat bordered below by narrow blackish line linking malars; ashy-grey neck side and breast shading whitish on belly and buffy on flanks; bill blackish, legs greyish-flesh; male non-breeding has breast more solidly grey, bill all black. Female is like male but facial pattern less distinct, chin whitish, shading to yellow-tinged buff on throat and breast (sometimes with partly ruby throat). Juvenile is very like juvenile Erithacus rubecula or Luscinia megarhynchos.
Systematics History
Subspecies
Distribution
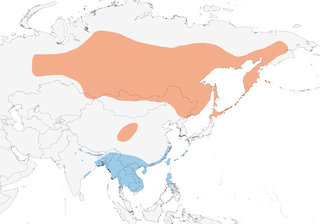
C Ural Mts and Siberia E to Anadyr, Kamchatka, Commander Is, S to N Mongolia, N Korea, N Japan and Kuril Is, also C China (NE Qinghai, SW Gansu, N Sichuan); winters in S & SE Asia, Taiwan and Philippines.
Habitat
Movement
Migrant, moving through Mongolia, China, Korea and Japan to winter quarters mainly in SE Asia from NE India E to Philippines. W populations probably move E initially, to avoid high mountain ranges, before turning S; passage migrant (may occur at 4000 m) and winter visitor, Sept–Apr, in E Himalayan foothills, straggling to Rajasthan and farther S. Exodus from breeding areas starts late Aug, complete by mid-Sept in N areas and by early Oct in Transbaikalia. Main autumn passage in Mongolia Sept, in NE China (Beidaihe) mid-Sept to mid-Oct; records in Hong Kong generally end Oct to end Apr, and in Philippines Oct to early May. Reportedly abundant in S Myanmar Nov–Jan. Passage in Japan mainly late Oct to mid-Nov and late Apr to early May. Arrives back in Mongolia and in S Russian breeding range middle to late May, but in N not until late May or early Jun. Casual winter visitor to Palau. Vagrants recorded Europe , North America (Alaska), Malaysia and New Guinea.
Diet and Foraging
Insects, including flies and their larvae, ants, wasps and beetles; also plant material. In May, China, 88·5% of food consisted of insects, including beetles and mosquitoes, the rest plant matter; stomachs of E Russian birds held insects (largely beetles) and their larvae, while birds on Sakhalin I took seashore amphipods (Gammarus). Food brought to nestlings comprised adult and larval hymenopterans, adult and larval beetles, adult and larval flies, spiders, molluscs, bugs, stoneflies, adult and larval lepidopterans, an adult dragonfly and a myriapod. Forages mainly on ground , taking items from hard surfaces; also gleans from lowest parts of reeds, grass clumps and bushes. In winter quarters, noted often to be most active in twilight. Males hold winter territories.
Sounds and Vocal Behavior
Song , started before spring departure for breeding grounds, a low, rapid, musical warble comprising squeaky, chortling, jangly silvery, metallic, harsh and a few clear musical notes, with much mimicry, “chil chil chil-li chilli” and so on; more melodious than that of Cyanecula svecica, sometimes considered as fine as L. megarhynchos song. Calls include short harsh nasal “ché”, “tshuk” or “chakh” in mild (probably territorial) agitation, sometimes followed by snatches of plaintive whistling song; short musical downslurred “svee-eek” or “chee-wee”, not unlike call of Larvivora cyane; harsh churr in alarm; in Japan commonest call is “cu-ééé”, second syllable higher and stressed, and elsewhere reported as loud whistling “tiuit-tiuit”, this sometimes combined with “chakh” call as “huiit-tak-tak”.
Breeding
Conservation Status
- Year-round
- Migration
- Breeding
- Non-Breeding