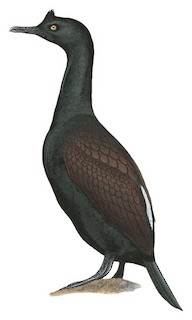
Bank Cormorant Phalacrocorax neglectus Scientific name definitions
- EN Endangered
- Names (24)
- Monotypic
Text last updated April 11, 2014
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Bankduiker |
| Bulgarian | Омаров корморан |
| Catalan | corb marí ullgroc |
| Czech | kormorán pobřežní |
| Dutch | Kustaalscholver |
| English | Bank Cormorant |
| English (United States) | Bank Cormorant |
| Finnish | matalikkomerimetso |
| French | Cormoran des bancs |
| French (France) | Cormoran des bancs |
| German | Küstenscharbe |
| Icelandic | Þangskarfur |
| Japanese | ハナグロウ |
| Norwegian | benguelaskarv |
| Polish | kormoran białorzytny |
| Portuguese (Angola) | Corvo-marinho das barrancas |
| Russian | Береговой баклан |
| Serbian | Namibijski vranac |
| Slovak | kormorán bledooký |
| Spanish | Cormorán de Bajío |
| Spanish (Spain) | Cormorán de bajío |
| Swedish | vitgumpad skarv |
| Turkish | Sahil Karabatağı |
| Ukrainian | Баклан береговий |
Phalacrocorax neglectus (Wahlberg, 1855)
Definitions
- PHALACROCORAX
- phalacrocorax
- neglecta / neglectum / neglectus
The Key to Scientific Names
Legend Overview
Field Identification
76 cm; male 1775–2425 g, female 1500–2150 g; wingspan 132 cm. Rather stocky cormorant with short erectile crest on anterior half of crown, thick neck, rather short tail and robust body, the head is fully feathered except for gular pouch and small bare area at base of mandible. Adult breeding has shiny black head, neck and underparts , the gloss appearing dull greenish, sometimes slighly purplish or bluish, with a few scatttered short white filoplumes on head, much more numerous but still rather scattered on hindneck; also shiny black on back to uppertail-coverts, the rump covered with dense white flecking forming conspicuous and often solid white patch ; mantle, scapulars and upperwing dark but have rather bronze-brown hue, each feather (except remiges) narrowly fringed blackish grey (visible at very close range), underwing black; tail dull black; underparts shiny black; later during breeding season, white filoplumes including patch on rump are lost; in non-breeding season duller and more uniform brownish black ; iris pale dull yellow, honey-coloured or dull brownish, with green to bluish crescent on lowest part; eyelids, bare skin at base of mandible and gular pouch black; bill blackish grey, often dull brownish at tip; legs black. Sexes similar. Juvenile entirely blackish brown, iris dark. Eye colour, black bare skin near gape and on gular region, and overall stocky appearance highly characteristic, meaning present species is unlikely to be confused with any other cormorant.
Systematics History
Subspecies
Distribution
Coast of C Namibia and W South Africa, from around Swakopmund to Cape Agulhas. Non-breeders recorded N to Hoanibmond (NW Namibia).
Habitat
Strictly marine, occupying cold waters of the Benguela Current. Prefers inshore waters, rarely fishing more than 10 km from land; range coincides with distribution of beds of kelp (Ecklonia maxima), where birds usually forage. Breeds on offshore islands, islets, stacks and rocky outcrops; occasionally on breakwaters and other human constructions; often near high-tide level and exposed to sea spray.
Movement
Adults are highly sedentary, and although individuals have been known to move up to 150 km, a capture-recapture survey recovered a large majority of adults within 10 km of the point of banding. Juveniles tend to disperse over larger distances, with adults and immatures tending to occur and forage in different areas# (1).
Diet and Foraging
Varied prey, including fish, crustaceans, cephalopods and molluscs. Across most of its range, prefers to forage on sea floor, especially among kelp beds at depths of 5–15 m, where it takes mainly klipfish (Clinidae) and blennies (Blenniidae). Cape rock lobster (Jasus lalandi) particularly important in diet in South Africa. However, in N of its range, in Namibia, where largest populations occur, the species forages away from kelp beds and most of its diet consists of pelagic gobies (Sufflogobius bibarbatus) typically foraged at depths of 30–40 m (2). It generally fishes alone or in small groups.
Sounds and Vocal Behavior
Mainly silent. Adult at nest utters a throaty plaintive, drawn-out alarm cry “rroaaauw”.
Breeding
Breeding activity occurs year-round but is concentrated in May–Oct in S of range, and in Nov–Apr in C Namibian colonies at N extent of its range (3). Normally nests in colonies of fewer than 100 pairs. Nests are built on exposed rocks, walls or artificial platforms near the sea, and are often lost in rough weather. Winter breeding in S Africa makes nests particularly susceptible to being washed away by storms, but may be offset by lower temperatures: elsewhere, high levels of chick mortality have been recorded during heatwaves (4). Nests mainly comprise green seaweed with some sticks and feathers, cemented together with excreta; regularly re-used and may grow very large, weighing up to 6 kg. Clutch usually two eggs (1–3); incubation 29–30 days; initially naked chicks develop sooty-black down with some white on head; leave the nest before able to fly properly, and so are particularly vulnerable to predation and disturbance at this stage. Age at first breeding is 2–3 years and mean longevity of breeders is estimated at six years (5, 6).
Conservation Status
ENDANGERED. Global population estimated at 8400 birds in 2006, including 2800 breeding pairs. Now breeds at 45 localities between Hollam’s Bird I, Namibia, and Quoin Rock, South Africa. Approximately 80–90% of breeding population is in Namibia, on Mercury and Ichaboe islands. Non-breeding range extends from just S of Hoanibmond S to Die Walle. Total population in late 1980s was c. 18,000 birds at some 47 colonies, including 12,800 on islands of Ichaboe and Mercury, Namibia. However, numbers have declined severely in recent years, by 4·3% per year in 1990–2006. Several populations in W & N Cape Province have declined and seven former breeding localities have been abandoned. Between 1993 and 1998, the Namibian breeding population is estimated to have declined by 68%, largely due to a population collapse on Ichaboe I after 1994/95, mainly thought to result from food shortage, but other factors, e.g., predation and displacement by seals may also be causes. Numbers on Ichaboe have since continued to decline, and although numbers have increased on Mercury I and are currently stable, the total Namibian population in 2006 was 39% smaller than in 1993 (7, 8). Diverse threats affect this species, with declines in prey availability now particularly important. Decreased abundance of goby off C Namibia in 1994 led to large declines on Mercury and Ichaboe Is; if goby are commercially harvested off Namibia, further significant declines are predicted in the species’s populations on these two islands (5). Numbers of breeders have also fallen in areas of South Africa in line with reduced abundance of Cape rock lobsters and the decline of this major food source may be another contributing factor (8). In South Africa, where Jasus lalandii is the principal prey, this lobster’s populations have been severely depleted by overfishing and perhaps by climate change, with recent movement of lobster populations away from NW coast of South Africa to the S & E, resulting in a mismatch between the distributions of breeding cormorants and their chief prey (2). Human disturbance has resulted in loss of four colonies, and reductions in populations at six others, between 1978 and 1997. Cape fur seals (Arctocephalus pusillus), which are major predators of fledged young, displaced 1824 pairs from Mercury I between 1978 and 1986. Despite expulsion of most seals, the colony has recovered poorly. Seals have caused declines at three other colonies, and occur at 16 breeding localities, often severely restricting breeding space (5). Oil spills are also a threat: 25% of population of Robben I was lost due to Treasure oil spill in 2000. Habitat destruction, both through guano collection and coastal development, has been a significant threat in past and guano collection contines on Ichaboe I. Alien mammalian predators, allowed access through the construction of a landbridge, caused the extinction of the Lambert’s Bay Bird I colony. Predation of eggs and chicks by Kelp Gull (Larus dominicanus) and Great White Pelican (Pelecanus onocrotalus) is also a problem. Avian cholera has affected other cormorant species in South Africa and could have catastrophic consequences for this species (5, 7). In South Africa, it is protected by law and major breeding islands are national parks, nature reserves or otherwise protected. However, some of the smaller breeding rocks are not protected and only 11 of 45 extant breeding colonies have nature reserve status (5). In Namibia, breeding islands are administered by the Ministry of Fisheries and Marine Resources, but just three are staffed.

- Year-round
- Migration
- Breeding
- Non-Breeding