Brown Teal Anas chlorotis Scientific name definitions
- NT Near Threatened
- Names (21)
- Monotypic
Text last updated May 17, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Bulgarian | Новозеландско бърне |
| Catalan | xarxet de Nova Zelanda |
| Czech | čírka novozélandská |
| Danish | Newzealandsk Brunand |
| Dutch | Bruine Taling |
| English | Brown Teal |
| English (United States) | Brown Teal |
| French | Sarcelle de Nouvelle-Zélande |
| French (France) | Sarcelle de Nouvelle-Zélande |
| German | Neuseelandente |
| Japanese | チャイロコガモ |
| Norwegian | kobberand |
| Polish | cyraneczka rdzawa |
| Russian | Бурый чирок |
| Serbian | Novozelandska krdža |
| Slovak | kačica hnedá |
| Spanish | Cerceta Maorí |
| Spanish (Spain) | Cerceta maorí |
| Swedish | brun kricka |
| Turkish | Pateke |
| Ukrainian | Чирянка каштанововола |
Anas chlorotis Gray, 1845
Definitions
- ANAS
- chlorotis
The Key to Scientific Names
Legend Overview
Field Identification
36–48 cm; male 586–860 g, female 530–860 g (1). Present species resembles wholly allopatric A. castanea of Australia, with breeding male having head and neck iridescent green , brown face with narrow white eyering , dark brown body with paler-barred flank feathers, dark chestnut breast, green speculum with white trailing edge to it, and black undertail (1); eclipse plumage similar to female, which some males also exhibit as breeding plumage. Female uniform dark brown, with paler breast and ventral region, and has conspicuous white eyering. Bare parts: bill black , legs and feet dark grey, and eye black (1). Juvenile resembles female, although generally darker prior to first moult and body and breast feathers have broad buff fringes, affording strongly mottled appearance (1). For differences from <em>A. aucklandica</em> and A. nesiotis, both of which are confined to New Zealand’s subantarctic islands, and which were previously treated as conspecific with present species, see Taxonomy comments and those species.
Systematics History
Subspecies
Hybridization
Hybrid Records and Media Contributed to eBird
-
Mallard x Brown Teal (hybrid) Anas platyrhynchos x chlorotis
Distribution
Great Barrier I and isolated areas of North I (Mimiwhangata Bay and Teal Bay) and SW South I (Fiordland), in New Zealand; formerly more widespread (including Chatham Is). Reintroduced at several locations (Coromandel, Tawharanui, Cape Kidnappers, Tuhua I), but uncertain as yet if these populations will survive.
Habitat
Coastal waters in sheltered bays and, inland, on marshes, pools and streams with some tree cover. Current range of habitat is much narrower than that prior to the arrival of humans, when forested ecosystems were also occupied (4).
Movement
Presumably sedentary; not recorded outside its now much-reduced range.
Diet and Foraging
Mostly aquatic invertebrates (insects and their larvae, crustaceans, molluscs). Forages by probing, dabbling, upending and diving near inlinemedia or, at low tide, on patches of kelp along shoreline. Often nocturnal, particularly where skuas (Catharacta) present. Flocking is reasonably common (1).
Sounds and Vocal Behavior
Male gives soft, high-pitched wheezy, bisyllabic whistles, “mmn-yea”, given when disturbed, during social courtship and antagonistic behaviour within flocks, as well as bell-like popping sounds, mostly during courtship (1); female emits low quacks and growls, mostly during social interactions or in contact with young, as well as high-pitched call of up to seven syllables (1).
Breeding
Mainly Jun–Oct, peak Jul–Aug, but broods recorded all months except Apr–May (1). Monogamous and single-brooded, although pairs may re-lay if first clutch is lost; male defends breeding territory (1). In single pairs; nest is a woven cup made of surrounding grass lined with down, situated on ground among vegetation, typically Carex or Cyperus near water, also in long grass, Typha stand or fern clump (1). Usually 5–6 eggs (3–9) (1), pale fawn; size 56·7–66·4 mm × 38·8–45·9 mm; incubation by female alone (although male remains close by) (1) c. 29–30 days (27–30 days in captivity) (1); chicks have brown down above , buffish below, with streaked face; fledging 50–55 days (captivity) or 60–70 days (wild) (1). Young cared for by both adults . Each pair raises on average 0·9–2 young to fledging, with annual adult survival of 0·63 and that of first-years 0·1 (1), but on E coast of Northland fledgling survival almost nil in mid 1990s, with just one of 51 identifiable fledglings surviving to recruit into one population and almost all fledgling mortality occurring within three months of independence; in this region, annual adult survival was 15% at Clendon Cove and 43% at Tutaematai, and most deaths occurred Oct–Dec, immediately post-breeding (5). On Great Barrier I eggs hatched in 74% of nests (n = 50), and 66% of eggs (n = 236) hatched, with 31 females fledging a total of just 15 young and most broods (72% n = 32) lost within ten days of hatching (6). Reaches sexual maturity when one year old (1), with annual survival of captive-bred birds introduced into conservation area varying between 48% and 85% (7). Has lived to 24 years in captivity (1). Natural predators include New Zealand Falcons (Falco novaeseelandiae) (8).
Conservation Status
Not globally threatened. Currently considered Near Threatened. CITES I. Restricted-range species: present in North Island of New Zealand EBA and South Island of New Zealand EBA. <em>A. chlorotis</em> was once common and widespread (4) on North I, South I, Stewart I (where last sighted in 1980, although the species was probably effectively extinct there by late 1940s) (9) and Chathams, but range now greatly reduced. Remnant populations now confined to a few sites in NW North I with small reintroduced populations on South I. Strongholds are on Great Barrier I, Northland and Coromandel. Great Barrier I held 1300–1500 individuals in early 1990s (when population assumed to be stable) (10), falling to c. 500 in first years of 21st century, then rising to 800 in 2013 BirdLife International (2016) Species factsheet: Anas chlorotis. Downloaded from http://www.birdlife.org on 17/05/2016. ; population on E coast of Northland at Mimiwhangata and Teal Bay, declined by 65% between 1988 and 1999 (11) (habitat destruction by cattle seeking moisture during droughts was significant cause of decline) (5) to c. 100 individuals in 2001, before increasing to almost 350 by 2007 and 500 by 2013 BirdLife International (2016) Species factsheet: Anas chlorotis. Downloaded from http://www.birdlife.org on 17/05/2016. ; in N Coromandel, where conservation efforts commenced in 2003 (7), reintroduced population numbered c. 500 individuals in early 2008, and c. 1000 by 2011 BirdLife International (2016) Species factsheet: Anas chlorotis. Downloaded from http://www.birdlife.org on 17/05/2016. . Study on Great Barrier I suggested that population was in rapid decline, this prompting intensive management, and numbers rose again. Small islands where previously introduced individuals persisted for up to two decades are possibly too small for long-term survival of chlorotis (some of these populations apparently now near extinction), but overall trend now positive. New populations recently established in N & E North I at Tawharanui, Tuhua I (Mayor I) and Cape Kidnappers, but long-term outcomes of these releases will need to be assessed. Following four translocations of captive-bred individuals of chlorotis during 2008–2010 at Tawharanui Open Sanctuary, in Auckland region of NW North I, a radio-telemetric study found that supplemental feeding, contrary to fears expressed by some, had no effect on post-release survival; it was concluded that such feeding provides a valuable and necessary conservation tool for this duck, particularly when releases are made in managed areas, in part because captive and captive-bred teal have much shorter and lighter small intestines and caeca than wild birds, and these differences could reduce the ability of captive-bred teal to efficiently digest a wild diet, and work is also still needed to prevent Purple Swamphens (Porphyrio porphyrio melanotus) from accessing supplemental food (12, 13). Formerly considered globally Endangered due to its reduced range, number of breeding locations, area and quality of habitat, and rapid population declines BirdLife International (2016) Species factsheet: Anas chlorotis. Downloaded from http://www.birdlife.org on 17/05/2016. ; intensive conservation management has successfully halted declines and numbers are now on the increase (number of mature individuals is thought to be > 1000), with several new populations being established, a situation which led to its downlisting to Near Threatened in 2015 BirdLife International (2016) Species factsheet: Anas chlorotis. Downloaded from http://www.birdlife.org on 17/05/2016. .
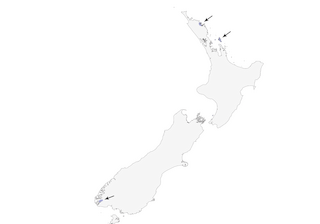
- Year-round
- Migration
- Breeding
- Non-Breeding