Cauca Guan Penelope perspicax Scientific name definitions
- VU Vulnerable
- Names (19)
- Monotypic
Text last updated September 13, 2015
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | guan del Cauca |
| Czech | guan kolumbijský |
| Dutch | Caucasjakohoen |
| English | Cauca Guan |
| English (United States) | Cauca Guan |
| French | Pénélope du Cauca |
| French (France) | Pénélope de la Cauca |
| German | Caucaguan |
| Japanese | アカシャクケイ |
| Norwegian | caucahokko |
| Polish | penelopa kasztanowata |
| Russian | Колумбийская пенелопа |
| Serbian | Kaukanski guan |
| Slovak | šuan gaštanový |
| Spanish | Pava del Cauca |
| Spanish (Spain) | Pava del Cauca |
| Swedish | caucaguan |
| Turkish | Kauka Guanı |
| Ukrainian | Пенелопа колумбійська |
Penelope perspicax Bangs, 1911
Definitions
- PENELOPE
- penelope
- perspicax
The Key to Scientific Names
Legend Overview
Introduction
Endemic to Colombia, the Cauca Guan is currently treated as Endangered by BirdLife International on account of it having a small and apparently declining range on the west slopes of the West and Central Andes. This guan is largely dependent on primary forest, although in some areas it has adapted to plantations, and is chiefly found at elevations between 900 and 2150 m. It is a medium-sized, drably colored cracid with bright red dewlap. The Cauca Guan is mainly dull brownish gray, except for the mainly chestnut rear underparts and tail; the feathers of the head to mantle and breast are scaled whitish. In addition to habitat destruction and fragmentation, which is ongoing, the Cauca Guan still apparently suffers from some direct human persecution, even within protected areas.
Field Identification
c. 76 cm; mean 1197 g (1). Back, rump, wings , belly and tail mainly rich chestnut ; belly and thighs have some inconspicuous darker markings. Heavily whitish-scaled feather edges from head to mantle and breast . Outer tail feathers darker than those of centre. Juvenile apparently undescribed, but presumably much like adult as in other Penelope (2). Larger P. purpurascens has conspicuously streaked (not scaled) appearance and olive-brown rear body, while smaller P. montagnii has tiny dewlap, and much duller chestnut lower back and wings.
Systematics History
Subspecies
Distribution
Scattered localities in W Colombia, on both slopes of W Andes and W slopes of C Andes; almost wholly confined to middle and upper Cauca Valley (Risaralda, Quindío, Valle del Cauca and Cauca). Much rarer on W slopes of W Andes, where seen mainly in low passes.
Habitat
Humid forest in upper tropical and subtropical zones, mainly between 1200 m and 2100 m, but has been recorded down to 816 m and as high as 2690 m (2). Occurs both in primary forest and second growth, and even in groves near tracks and plantations of exotic trees such as Chinese ash Fraxinus sinensis (Oleaceae), where the species has locally been recorded at very high densities seasonally (3), and Pinus patula (Pinaceae) (2), but usually only if large fragments of mature forest exist close by (2). Nonetheless, the species does appear to be able to breed in suboptimal habitat (2). Spends most time in middle and upper strata (> 10 m above the ground), but descends lower especially at edges (2).
Movement
No information available; presumably sedentary.
Diet and Foraging
Mid-1990s study of diet revealed that 97·1% of feeding observations related to 13 species of fruiting plants , which in numerical importance were: Cecropia spp. (Urticaceae), Faramea sp. (Rubiaceae), Stylogyne sp. (Myrsinaceae), Guettarda crispiflora (Rubiceae), Ficus obtusifolia, Ficus sp. (Moraceae), Siparuna aspera (Siparunaceae), Clusia sp. (Clusiaceae), Miconia coronata (Melastomataceae), Inga sp. (Fabaceae) and Brunellia comocladifolia (Brunelliaceae) (2); other species recorded through incidental observations include Dendropanax sp. (Araliaceae ), the exotic Chinese ash Fraxinus sinensis (Oleaceae) and Solanum sp. (Solanaceae); the ash, planted as part of a revegetation programme, is considered to be very important seasonally (3). An additional study, in early 2000s, at a second locality, yielded records of fruits of 89 species (pertaining to many of same families), sometimes when not even ripe (Symplocos quindiuensis, Symplocaceae, Turpinia occidentalis Staphylaceae, and Vismia guianensis, Clusiaceae), but also leaves of four species and foliage of two, in a ratio of 67·5%, 26·8% and 1·1%, respectively (4). Same study revealed concentration on certain high-protein fruits during breeding period, despite abundance of fruiting plants of broad range of species, and that Fraxinus leaves (both young and, especially, mature) were preferentially selected during Sept–Dec, when other foods were generally scarce (4). Apparently also ingests small pebbles (2). Young in captivity eat many insects, and adults (typically in groups of up to seven) also recorded taking invertebrates flushed by Labidus praedator army ants, principally between Apr and Sept (1, 5). Forages in flocks of up to 30 birds (1), more usually 3–6 birds (2), sometimes with other species of birds (e.g. cotingas and toucans) and mammals, from low trees and shrubs almost to canopy, mainly 2–20 m above ground level (1).
Sounds and Vocal Behavior
In general, like other guans, loud, raucous honking, especially during breeding season and when alarmed. Few published details: ‘song’ (sometimes given chorus) is a very loud, repeated “chiriwichi, chiriwichi, chiriwichi...” mainly given in early morning and especially between Feb and Jun; in contact gives a soft downslurred whistle, which is used to regroup flock members following disturbance; and utters a rapid series of loud “quan...quan” notes in alarm , which sometimes speed-up noticeably, e.g. when fleeing human observer (2). Young maintain contact with adults using a sibilant “piiiiuuuu, piiiuuuu”, which starts loudest (1).
Breeding
Breeding apparently coincides with wet seasons and period of peak fruit abundance, suggested to be in Sept–Oct and Feb–Mar (2) or mainly (Dec)Jan–Jun (with young from Mar) (1); nest in Feb, of dry leaves and hidden by ferns in Pinus patula (Pinaceae) stump c. 1·8 m above ground; another in Sept, of thin branches lined with dry leaves, surrounded by ferns and lianas on fallen Myrcia (Myrciaceae) tree (2); and one in Dec was c. 1 m above ground, constructed of leaves and stems of a Gleichenia sp. fern and had outside dimensions of 34 cm (diameter) by 25 cm (depth) (1); one juvenile, one-third grown, collected in Mar. Clutch two white eggs, size 73·9–78·9 mm × 49–53·4 mm, mass 94–96 g (2, 1), incubated by female alone, 29 days in captivity (2). Chick similar to that of P. montagnii, has black crown with white superciliary, dark wings with pale bars on wing-coverts, rufous mantle and breast, pale throat and white belly (2). Young adult-like at c. 5 months (2), but remain with adults for c. 1 year (1). No further information available.
Conservation Status
ENDANGERED. Populations largely protected, but vigilance needed. Historical range of 24,900 km² reduced to just 750 km², and this heavily fragmented (3). Considered not uncommon in early 20th century, but much scarcer since then, mainly as a result of habitat loss; total population now thought to number fewer than 1000 individuals. Prior to mid 1980s, scarcity of recent records and almost complete destruction of forest in middle Cauca Valley led to belief that species was possibly extinct, or very nearly so. However, observations and capture of live birds showed that small but stable populations remained, at least in a few areas. In mid 1990s, largest numbers were apparently in SE Risaralda and N Quindío, especially in Ucumarí Regional Park (4240 ha, where density apparently quite high, with 16 birds counted over two days in 1990), Otún-Quimbaya Flora and Fauna Sanctuary (489 ha, population 144–264 individuals) (3) and Bremen Forest Reserve (2). Also receives protection in Bosque de Yotoco Reserve (559 ha, population estimated at 35–61 birds) (3), where squatters have been effectively excluded; known only from fringes of Munchique National Park and not seen there recently, although extensive forest remains. Recently observed in La Sirena reserve and Chorro de Plata, a private reserve on outskirts of Cali (3). Forest destruction is main threat, but hunting has probably also contributed to decline; poaching is widespread in Ucumarí Regional Park and Munchique National Park. Captive-breeding programme underway at Cali Zoo (2).
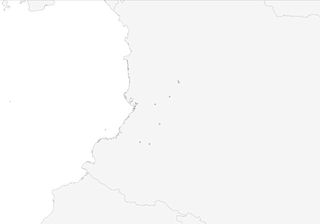
- Year-round
- Migration
- Breeding
- Non-Breeding




























