Eurasian Coot Fulica atra Scientific name definitions
Text last updated September 5, 2015
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Albanian | Bajza |
| Arabic | غرة أوراسية |
| Armenian | Սև փարփար |
| Assamese | নল ঢেকৰ |
| Asturian | Gallarñn comñn |
| Azerbaijani | Adi qaşqaldaq |
| Basque | Kopetazuri arrunta |
| Bulgarian | Лиска |
| Catalan | fotja comuna |
| Chinese | 白冠雞 |
| Chinese (Hong Kong SAR China) | 白骨頂 |
| Chinese (SIM) | 骨顶鸡 |
| Croatian | liska |
| Czech | lyska černá |
| Danish | Blishøne |
| Dutch | Meerkoet |
| English | Eurasian Coot |
| English (United States) | Eurasian Coot |
| Faroese | Sjógvhøna |
| Finnish | nokikana |
| French | Foulque macroule |
| French (France) | Foulque macroule |
| Galician | Galo de auga común |
| German | Blässhuhn |
| Greek | (Κοινή) Φαλαρίδα |
| Gujarati | ભગતડું |
| Hebrew | אגמית מצויה |
| Hindi | टिकड़ी |
| Hungarian | Szárcsa |
| Icelandic | Bleshæna |
| Indonesian | Mandar hitam |
| Italian | Folaga |
| Japanese | オオバン |
| Korean | 물닭 |
| Latvian | Laucis |
| Lithuanian | Laukys |
| Malayalam | വെള്ളക്കൊക്കൻ കുളക്കോഴി |
| Marathi | वारकरी |
| Mongolian | Халзан түнжүүр |
| Norwegian | sothøne |
| Persian | چنگر معمولی |
| Polish | łyska |
| Portuguese (Portugal) | Galeirão-comum |
| Punjabi (India) | ਆਰੀ |
| Romanian | Lișiță |
| Russian | Лысуха |
| Serbian | Liska |
| Slovak | lyska čierna |
| Slovenian | Liska |
| Spanish | Focha Común |
| Spanish (Spain) | Focha común |
| Swedish | sothöna |
| Telugu | నల్ల బొల్లి కోడి |
| Thai | นกคู้ต |
| Turkish | Sakarmeke |
| Ukrainian | Лиска звичайна |
Fulica atra Linnaeus, 1758
Definitions
- FULICA
- fulica
- atra
The Key to Scientific Names
Legend Overview
Field Identification
36–39 cm; nominate male 610–1200 (902) g, female 610–1150 (770) g; race australis male 481–660 (568) g, female 476–609 (552) g, unsexed 305–725 (511) g; wingspan 70–80 cm in nominate , 56–64 cm in australis. Distinguished from F. cristata by greater contrast between black head and neck and somewhat paler body, white tips to secondaries (nominate and lugubris), lack of red knobs at top of frontal shield , and pointed projection of loral feathering between bill and shield. Differs from <em>F. americana</em> in colours of bill, shield, legs and feet , dark undertail-coverts, and less contrast between dark head and paler body. Sexes alike, female averages smaller. Immature like adult but upperparts more washed with olive brown and feathers of throat, cheeks and underparts with broader white tips, giving variably mottled appearance; attains adult bare part colours between autumn and spring. Juvenile has crown, hindneck and upperparts dark brown to dark olive brown, rest of head and neck mixed dark grey and white (whitest on throat), flanks olive brown, breast whitish and belly to vent white with grey suffusion; iris dark grey to pale brown; bill dusky grey, pink towards tip in australis; legs and feet grey. Races separated on: amount of white on tips of secondaries, much reduced or absent in novaeguineae and australis; size, nominate larger than lugubris; and underpart colour, darker in novaeguinea than australis; <em>novaeguinea</em> has deeper black head and neck.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Closely related to F. cristata, F. alai, F. americana, F. leucoptera and F. ardesiaca. Described form langkensis (from Sri Lanka) included in nominate race; anggiensis (from Arfak Mts, in NW New Guinea) regarded as a synonym of lugubris. Four subspecies recognized.Subspecies
Fulica atra atra Scientific name definitions
Distribution
Fulica atra atra Linnaeus, 1758
Definitions
- FULICA
- fulica
- atra
The Key to Scientific Names
Legend Overview
Fulica atra lugubris Scientific name definitions
Distribution
Fulica atra lugubris Müller, 1847
Definitions
- FULICA
- fulica
- atra
- lugubris
The Key to Scientific Names
Legend Overview
Fulica atra novaeguineae Scientific name definitions
Distribution
Fulica atra novaeguineae Rand, 1940
Definitions
- FULICA
- fulica
- atra
- novaeguinae / novaeguineae / novaeguineensis
The Key to Scientific Names
Legend Overview
Fulica atra australis Scientific name definitions
Distribution
Fulica atra australis Gould, 1845
Definitions
- FULICA
- fulica
- atra
- australe / australis
The Key to Scientific Names
Legend Overview
Hybridization
Hybrid Records and Media Contributed to eBird
-
Eurasian Moorhen x Eurasian Coot (hybrid) Gallinula chloropus x Fulica atra
-
Eurasian x Red-knobbed Coot (hybrid) Fulica atra x cristata
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Mainly large, still or slow-moving waters . Inhabits lakes, pools, ponds, reservoirs, barrages, gravel pits, canals, drainage channels, dykes, rivers, creeks, oxbow lakes, open marshes, floodlands and lagoons; also lakes and pools in towns, and salt-pans and clay-pans. Exploits temporary pools and seasonal marshes for breeding. Prefers fairly shallow waters with room to dive and with muddy bottom well furnished with marginal, emergent, floating or submerged vegetation; requires some open water but when breeding not normally far from banks or from emergent or floating vegetation. In winter will resort to quiet estuarine or inshore sea waters, and often occurs on open water of lakes and reservoirs. Avoids small pools and streams; sometimes occurs on fast rivers where suitable vegetation flourishes. Makes little use of cover, either in water or on land. Normally occurs in lowlands, but up to 1000 m in Europe, 2000 m in Tadjikistan and Iran, 2500 m in Kashmir; occurs up to 3500 m in New Guinea, where resident at montane lakes.
Movement
Present all year in warm and temperate regions, although not necessarily resident, but mainly migratory in N Eurasia under influence of continental climate. In W Palearctic, N populations winter from North Sea, Baltic, EC Europe, Black and Caspian Seas, Iraq and Arabia S to Senegal, Mali, N Nigeria, S Niger, WC Chad, desert oases in Morocco and S Algeria, and N Sudan (along R Nile). Movement through continental Europe on broad front, W to S, and coastal movement via Baltic brings birds from as far E as Moscow into North Sea area; S movements occur Sept–Nov and return passage Mar–May. Crosses Sahara on broad front, reaching W African wintering areas Nov and departing Mar–Apr. Birds wintering Black and Caspian Seas S to Iraq are presumably from former USSR; movements noted Azerbaijan Sept–Dec and Feb–Apr. Moult migrations occur; little studied but some involve non-breeding adults, while moulting concentrations occur Jun–Sept in Denmark, Bavaria, Bodensee, Black Sea, and probably much more widely. Further E in Asia, N populations winter on Indian Subcontinent, with N passage observed in Afghanistan and Pakistan, Feb–May; also in SE Asia S to Thailand, Cambodia, Vietnam and Philippines (recorded Nov–Mar); and E to S Japan and Ryukyu Is, stragglers reaching Northern Mariana Is. Dispersive in Australia, where large changes in abundance occur, possibly in response to weather conditions and changes in water levels, as birds move to flooded areas, breed and then depart; such patterns may not be seasonal, but seasonal changes occur in some areas; in New Zealand, numbers may fluctuate seasonally. Probably often crosses Bass Strait and Tasman Sea; also crosses other oceans, and recorded occasionally on Norfolk I, Lord Howe I and Macquarie I. Birds seen irregularly in New Guinea lowlands may be vagrants from Australia. Accidental in Alaska (Nov 1962), Labrador (Dec 1927), Newfoundland, Quebec (Dec 1995), Greenland (c. 10 records) (3), Iceland (occasionally breeds), Spitsbergen and Faeroes.
Diet and Foraging
Omnivorous, but primarily vegetarian . Eats mainly vegetative parts and seeds of aquatic and sometimes terrestrial plants , including algae (Chara, Vaucheria, Cladophora, Spirogyra, Ectocarpus and Nostoc), vegetative parts of Potamogeton, Ruppia, Zannichella, Elodea, Zostera, Vallisnaria, Lemna, Ceratophyllum, Ranunculus, Polygonum, Myriophyllum, Najas, Scirpus, Typha, Phragmites, Phalaris, Sparganium and grasses; also seeds of Ruppia, Sparganium, Potamogeton, Carex, Nymphaea, grasses and cereals. Takes plant debris drifting on water surface. Animal food eaten includes worms, leeches, molluscs, shrimps, adult and larval insects (especially Diptera, Trichoptera, Odonata, Lepidoptera, Coleoptera and Hemiptera), spiders, fish, fish eggs, frogs, birds and their eggs, and small mammals. Takes fish food and duck food. Employs diverse foraging methods (see Family Text), including scraping algae from hard substrates, gleaning, dabbling, upending , diving and grazing. Feeds in flocks on land, grazing near water , especially when wind causes high waves. Kleptoparasitizes conspecifics as well as swans and ducks. Large food items are shaken to break them up, or may be chopped up by bill on ground. Diurnal , but often active at night on moonlit nights or on floodlit waters.
Sounds and Vocal Behavior
Combat call of male a sharp, explosive “pssi”, of female a short, croaking “ai”; alarm call of male a sharp variant of combat call, of female a rapid sequence of “ai-oeu” sounds. Typical contact call a single, short “kow”, “kowk”, “kup”, or sharper “kick”; sometimes 2 notes combined, e.g. “kick-kowp”; male also has mechanical “p” or “ta” calls and female a high, short, falsetto “oeu”. Calls often metallic, resonant, querulous or explosive, particularly sharp and high when birds agitated. Often noisy.
Breeding
Europe, Feb–Sept, mostly Mar–Jul; Azerbaijan, Apr–Jun; N Africa, Mar–Jun; Kashmir and N India, May–Sept; S India, Nov–Dec; Australia, mainly Aug–Feb, also other months; New Zealand, Sept–Feb. Gregarious, but monogamous, territorial and pugnacious when breeding. Some birds territorial all year where climate and food availability permit; pair-bonds may sometimes be retained in flocking and migratory populations. Nest almost always in shallow water, normally in emergent vegetation but sometimes in open; normally resting on bottom or trampled foundation of vegetation, occasionally floating; artificial platforms, rafts, tree stumps, tree forks, or islands sometimes used. Receding water level can leave nest some way from water. Nest bulky , of dead and live stems and leaves, sometimes also twigs, bark and roots; external diameter 25–55 cm, height 8–35 cm; cup diameter 16–30 cm, depth 6–13 cm; nest may be built up if water level rises. Both sexes build; brood platforms for family also built by male. Usually 6–10 eggs (1–14), larger clutches may be laid by two females; eggs laid daily, sometimes at intervals of 1–2 days; up to three replacements laid after egg loss; incubation 21–26 days; by both parents; hatching asynchronous ; black downy chick has orange to yellow tips to down of neck and side of head , wings and mantle, and red tips on lores and around shield, iris hazel to grey-brown, bare skin of crown red and blue, bill and shield red, bill white distally and black at tip, legs and feet slate grey; chicks precocial and nidifugous ; brooded on nest for 3–4 days; fed and cared for by both parents, which may divide brood temporarily or permanently; older young help feed chicks of later broods; young begin to dive at 3–5 weeks; become self-feeding at 30 days; fledging 55–60 days; fed by parents for up to two months; independent at 6–8 weeks; fly at 8–11 weeks; remain in parental territory for up to 14 weeks, possibly helping in territory defence. Age of first breeding one year (usually older). May have two broods per season.
Conservation Status
Not globally threatened (Least Concern). Nominate race has expanded range in Europe since late 19th century; marked population fluctuations in many areas due to hard winters, but has probably increased generally, aided by eutrophication, new man-made habitats, and adaptation to urban environments. In 1970s, populations of 40,000–100,000 pairs estimated for several European countries, while Jan counts for NW Europe totalled 438,000–646,000 birds, and European sector of former USSR held over 580,000 pairs in summer and 804,000 birds in winter; area of Mediterranean and Black Seas held 1,035,000–1,296,000 birds in 1969–1971 winters. Declining in Azerbaijan due to hunting and oil pollution. Locally common breeder in N Africa. Formerly abundant in winter, India and Pakistan; present status not clear. Uncommon and local New Guinea; increasing Japan; occurs only rarely in Borneo, Bali and Java; formerly bred in Java but no recent records. Widespread in Australia and New Zealand; locally common in Australia, counts in Victoria in 1991/92 totalling 110,000 and 131,000 birds, with populations stable. Displaced by wetland drainage but rapidly colonizes suitable artificial habitats such as dams, while in arid areas artificial wetlands act as refuges.
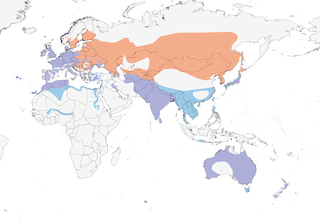
- Year-round
- Migration
- Breeding
- Non-Breeding































