Green Imperial-Pigeon Ducula aenea Scientific name definitions
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Assamese | পৰ্ঘুমা |
| Catalan | colom imperial verd |
| Chinese (SIM) | 绿皇鸠 |
| Czech | holub kovový |
| Dutch | Groene Muskaatduif |
| English | Green Imperial-Pigeon |
| English (United States) | Green Imperial-Pigeon |
| French | Carpophage de Pauline |
| French (France) | Carpophage de Pauline |
| German | Bronzefruchttaube |
| Indonesian | Pergam hijau |
| Japanese | ミカドバト |
| Malayalam | മേനി പ്രാവ് |
| Norwegian | bronsekeiserdue |
| Polish | muszkatela miedziana |
| Russian | Бронзовый плодовый голубь |
| Serbian | Zelenokrili golub |
| Slovak | plodožer bronzový |
| Spanish | Dúcula Verde |
| Spanish (Spain) | Dúcula verde |
| Swedish | grön kejsarduva |
| Thai | นกลุมพูเขียว |
| Turkish | Yeşil Has Güvercin |
| Ukrainian | Пінон малазійський |
Revision Notes
Brooke K. Keeney standardized the account with Clements taxonomy. Peter Pyle contributed to the Plumages, Molts, and Structure page. Shawn M. Billerman contributed to the Systematics page.
Ducula aenea (Linnaeus, 1766)
Definitions
- DUCULA
- aenea
The Key to Scientific Names
Legend Overview
Introduction
The Green Imperial-Pigeon has a broad distribution ranging from northern India and Nepal in the north, to the Philippines and Indonesia in the south. It is characterized by its vinous-gray head and underparts, metallic-green upperparts with some blue iridescence and bronzy reflections, and chestnut undertail coverts. Given such a widespread distribution, encompassing many islands, it is unsurprising that many subspecies have been described, although a good number have subsequently been synonymized, and most recent commentators have suggested that further rationalization may be desirable. On the other hand, several subspecies are comparatively distinctive, and the Nicobar Imperial-Pigeon (Ducula nicobarica) and Enggano Imperial-Pigeon (Ducula oenothorax) were recognized as full species. Local declines and even some insular extinctions have been reported, but in general, the Green Imperial-Pigeon remains a reasonably common and easily encountered species across large parts of its range, where it displays a broad affinity with all manner of wooded habitats at generally lower elevations (rarely exceeding 1,000 m), and a degree of acceptance of human-modified areas.
Field Identification
The Green Imperial-Pigeon is a medium-sized, sexually monochromatic Ducula, chiefly characterized by the pale vinous-gray head and underparts, dark metallic-green upperparts, wings, and tail, the latter without any pale bands, no contrast in the underwing in flight, dark chestnut undertail coverts, and (in some subspecies) a very striking chestnut nape patch.
Similar Species
There is some risk of confusion with several more restricted-range congenerics.
The Gray Imperial-Pigeon (Ducula pickeringii), which is found on islands off of northern Borneo, the extreme southern Philippines, and several islands between there and Sulawesi, is smaller and shows only a faint green gloss to the largely gray upperparts. Furthermore there is little, if no, contrast between the buffy-gray undertail coverts and the rest of the vinous-gray underparts, the underside of the tail is gray, and the cere is also gray.
The larger Elegant Imperial-Pigeon (Ducula concinna), which is a small-island specialist, has paler creamy-white underparts, head, and neck, slightly paler-colored undertail coverts, darker metallic-blue upperparts and tail (with hardly any green gloss), black underwing coverts, a yellow iris, and a swollen cere, which gives the effect of a very shallowly sloping forehead.
In mainland Asia, Sumatra, Borneo, and West Java, the Mountain Imperial-Pigeon (Ducula badia) is, as its name suggests, typically found at higher elevations than the present species, and it is also larger, with gray or deep maroon-colored upperparts, pale buff undertail coverts, and a broad gray tail-band contrasting with a black base to the rectrices.
On the Andaman Islands, the smaller Andaman Wood-Pigeon (Columba palumboides) is a potential confusion risk, especially at longer distances, although their flight is less ponderous and on less rounded wings. In closer views, the metallic green on the upperparts is restricted to the upper mantle and wing coverts fringes, while the tail lacks any metallic elements, and the throat, breast, and undertail coverts show a different pale-dark-pale contrast.
In areas of overlap, the Metallic Pigeon (Columba vitiensis), of Borneo, the Philippines, and the Lesser Sundas, has green fringes alone to the wing coverts, a much darker gray head and underparts (reaching as far as the undertail coverts), furrowed neck feathers, and orange iris, and much more conspicuous circum-orbital skin.
Plumages
Green Imperial-Pigeon s have 10 full-length primaries (numbered distally from innermost, p1 to outermost, p10), 10 secondaries (numbered proximally from outermost s1 to innermost s7 and including 3 tertials, numbered distally from t1 to t3), and 12 rectrices (numbered distally, from innermost r1 to outermost r6 on each side of the tail). Geographic variation in appearance is moderate to extensive; the following applies to the widespread subspecies D. a. sylvatica (see Systematics: Geographic Variation for descriptions of other subspecies) and is based on the descriptions of Baptista et al. (1) and Wells (2), along with examination of images at the Macaulay Library. See Molts for molt and plumage terminology. Definitive appearance is assumed at Formative or Second Basic Plumage; sexes similar in all plumages although showing slight average differences in Definitive Basic Plumage (see below). See Higgins and Davies (3) and Pyle et al. (4) for information on ageing of species within Ducula.
Natal Down
Undescribed in Green Imperial-Pigeon. In at least some other Ducula imperial-pigeons, natal down in hatchlings is pinkish tan.
Juvenile Plumage
Juvenile Plumage is similar to Definitive Basic Plumage but duller, the head and breast being grayer; back and upperwing coverts duller, sometimes with darker greenish fringes creating mottled or scaled appearance; pale terminal tail band only weakly developed. As in other Ducula imperial-pigeons, primaries, secondaries, and rectrices are narrower and more rounded at the tips than in later plumages (4).
Formative Plumage
Formative Plumage resembles Definitive Basic Plumage but individuals may undergo incomplete Preformative Molts and can be identified by retained juvenile wing and/or tail feathers. Juvenile outer primaries, outer rectrices, and/or (especially) secondaries among s3-4 and s7-s9 can be retained, narrower, browner, and contrastingly worn, distinguishing formative birds from those in Definitive Basic Plumage with retained basic secondaries (see below).
Definitive Basic Plumage
Head, neck and underparts pale gray-pink, some creamy-white feathers around base of bill forming an inconspicuous band; lores and crown generally more vinous gray, the nape and neck are somewhat grayer, and the chin and base of the moustachial area whitish, becoming pale pinkish on the throat. Upperparts (including back, scapulars, and lesser and median coverts) iridescent green with bluish or bronzy reflections (often reflecting different states of wear), becoming less glossy green over the back and uppertail coverts; rectrices blackish glossed dark blue-green from above, more prominently on the central pair (r1), and blackish brown from below. The greater coverts and tertials are rather less glossy green, and possess a gray cast, more so when worn. Primaries and secondaries blackish, with slight green or blackish-green iridescence on the outer webs of the secondaries from above. The vent, thighs, and femoral feathering are all rather paler and grayer than the rest of the underparts and the undertail coverts are dark chestnut. The underwing is pale gray, becoming darker and browner on the underside of the flight feathers. Definitive basic Females average slightly duller than Males, the the head and breast with less pink and the upperparts duller green, but overlap in plumages between the sexes appears to occur.
Definitive Basic Plumage is characterized by broader, more truncate, and fresher flight feathers than in Juvenile Plumage. Primaries show molt clines from more worn inner feathers to fresher outer feathers, and may also show two or more sets of feathers reflecting Staffelmauser or stepwise molt (see Molts), the latter indicating Definitive Basic Plumage as opposed to Formative Plumage (4). Secondaries likewise show fresher feathers medially, reflecting molt sequence, or may show mixed patterns of fresh and worn basic feathers, showing slight contrasts in wear but not shape, following Staffelmauser.
Molts
General
Molt and plumage terminology follows Humphrey and Parkes (5) as modified by Howell et al. (6). Under this nomenclature, terminology is based on evolution of molts along ancestral lineages of birds from ecdysis (molts) of reptiles, rather than on molts relative to breeding season, location, or time of the year (see 7,8for more information). As in other Ducula imperial-pigeons, Green Imperial-Pigeon exhibits a Complex Basic Strategy (cf. 6, 9), including incomplete-to-complete preformative and definitive prebasic molts but no prealternate molts (3,4).
Prejuvenile (First Prebasic) Molt
Complete, in the nest. No information. Presumably completed or near-completed by fledging.
Preformative Molt
Variation in extent not well documented in Green Imperial-Pigeon but examination of Macaulay images suggests that it can be incomplete or complete as in other Ducula imperial pigeons (4). When incomplete, outer primaries (among p9-p10), more frequently 1-5 secondaries among s3-s4 and s7-s9, and one or more juvenile rectrices may be expected to be retained.
Definitive Prebasic Molt
Incomplete to complete. In the Peninsular Malaysia, molt of wing feathers occurs between February and October (10). Primaries replaced distally (p1 to p10), secondaries replaced proximally from s1 and distally from the tertials (often bilaterally from the second tertial, t2), and rectrices generally replaced distally (r1 to r6) on each side of tail, with some variation in rectrix sequence possible. Following incomplete molts, Staffelmauser or stepwise molt (see 11), in which two or more waves of primaries can molt simultaneously, can result in some individuals, as in other Ducula imperial-pigeons (12, 10, 4).
Bare Parts
Bill
In adults, bluish gray or lead gray with a red or purplish cere (10, 13).
Iris and Facial Skin
Iris is dark brown to red with lilac (10, 13). In adults, outer ring and crimson orbital skin, duller and browner in juveniles.
Tarsus and Toes
In adults, dark pinkish red, or dull purple red (10, 13); paler pink in juveniles.
Measurements
Linear Measurements
Overall length 48–48 cm (male), 40.5–44.0 cm (female) (13), but for males Rasmussen and Anderton (14) gave 38–39 cm (D. a. sylvatica), 34–36 cm (D. a. pusilla), and 35–37 cm (D. a. andamanica), while for females their respective values were 37–38 cm (D. a. sylvatica), 34–35 cm (D. a. pusilla), and 34–35 cm (D. a. andamanica).
Linear measurements (in mm, from Gibbs et al. 13):
| Males | Females | |
| Wing length | 223–250 | 225–236 |
| Tail length | 120–146 (both sexes) | |
| Bill length | 23–25 (both sexes) | |
| Tarsus length | 26–31 (both sexes) |
Mass
Systematics History
Often considered conspecific with Enggano Imperial-Pigeon (Ducula oenothorax), though formerly considered specifically distinct (see 18). In their assessment of the taxa, del Hoyo and Collar (19), using the Tobias et al. (20) criteria, from which the numbers in brackets are derived, found that Ducula aenea differs in its gray versus purplish-pink [3]; chestnut versus green vent [3]; and duller, more emerald-green and bronzy reflectant upperparts [1]. Additional work is needed to confirm the taxonomic assessment of Ducula oenothorax, but the morphological differences between these two taxa are as strong as those in other undisputed species pairs in Ducula.
Subspecies paulina also sometimes considered a separate species, differing in its large, pale vinous-rufous nuchal patch (from hindneck to mantle and neck-sides, and connecting vaguely to postocular area), and slightly more pinkish-buffy face than other taxa.
Geographic Variation
Most described variation revolves around rather subtle differences in size, intensity of the metallic gloss above, the shade of gray on the head and underparts, and the extent of the paler throat, with more striking chestnut-naped forms in the northern Philippines and on Sulawesi, and an unusually darker saturated taxon off western Sumatra.
Subspecies
Owing to confusion over type locality of nominate aenea, previously thought to be in Malaysia but later corrected to Philippines (21), population now listed under polia was formerly included under aenea; proposed taxa chalybura and glaucocauda are synonyms of aenea (22). Subspecies variation not altogether clear: proposed form kwangtungensis (23) (southern China) included in sylvatica, while proposed taxa mista (Simeulue), babiensis (Babi and Lasia Islands, off southern Simeulue), and vicinus (Batu and Mentawai Islands; note spelling 24) included in consobrina (25, 18); pallidinucha (Tobea Island, off SE Sulawesi), pulchella (Togian Islands), sulana (Sula Islands) and intercedens (Peleng, in Banggai Islands) are all included in paulina (26), although it has recently been suggested that pulchella may, after all, represent a valid taxon (it shows a brown shoulder) (27); further analysis may reduce still further the number of subspecies accepted (19).
Green Imperial-Pigeon (Green) Ducula aenea [aenea Group]
Distribution
North India through Nepal and Bangladesh to southern China (including Hainan Island), south to southern Myanmar (central Tenasserim), northern and southern Thailand, Laos, Vietnam, and Cambodia.
Ducula aenea sylvatica (Tickell, 1833)
Definitions
- DUCULA
- aenea
- sylvatica / sylvaticum
The Key to Scientific Names
Legend Overview
Distribution
Southern India and Sri Lanka.
Ducula aenea pusilla (Blyth, 1849)
Definitions
- DUCULA
- aenea
- pusilla
The Key to Scientific Names
Legend Overview
Distribution
Andaman Islands.
Identification Summary
Compared to D. a. sylvatica and D. a. pusilla, has slightly more white around the face (chin and central throat) and a darker vent. Also said to be smaller than D. a. sylvatica (wing 229–236 mm) with metallic blue and even bronze reflections on the upperparts. Tail of male 145–150 mm, tail of female 145–150 mm (14).
Ducula aenea andamanica Abdulali, 1964
Definitions
- DUCULA
- aenea
- andamana / andamanensis / andamanica / andamanicus / andamanus
The Key to Scientific Names
Legend Overview
Ducula aenea consobrina Scientific name definitions
Systematics History
Some modern authorities prefer to maintain the subspecies D. a. babiensis and D. a. vicinus, based on the former’s larger size, and latter’s smaller size and darker pink head and breast (28).
Distribution
Islands off western Sumatra (excluding Enggano).
Identification Summary
Very like D. a. sylvatica, but with a larger bill, the white band around the bill reduced and suffused gray, and the head and underparts less obviously vinous. Also reported to have darker chestnut vent and more sharply delineated border between neck and back compared to neighboring subspecies (28). Wing 225–248 mm; bill 24 mm (13).
Ducula aenea consobrina (Salvadori, 1887)
Definitions
- DUCULA
- aenea
- consobrina
The Key to Scientific Names
Legend Overview
Distribution
Central and southern Malay Peninsula, the Greater Sundas, and the Lesser Sundas (east to Alor).
Identification Summary
Very similar to D. a. sylvatica, but green upperparts have stronger bronzy reflections, with a whiter chin and throat, more vinous ear-coverts, and remaining underparts are very slightly paler, with a less intense pinkish wash. Wing 232–247 mm. There is evidence of widespread intergradation with D. a. sylvatica (13).
Ducula aenea polia (Oberholser, 1917)
Definitions
- DUCULA
- aenea
- polia
The Key to Scientific Names
Legend Overview
Distribution
Western and southwest Philippines (the Calamian Group, Linapacan, Palawan, Dumaran, and Balabac), and Banggi (off northeast Borneo).
Identification Summary
Reportedly differs from D. a. polia in having a darker and bluer tail (but this feature is also said to be very variable), the chin and throat are often darker (more vinous-gray), and the gray hindneck is more clearly demarcated from the green mantle. Even more similar to the nominate (which see). Wing 241–253 mm.
Ducula aenea palawanensis (Blasius, 1888)
Definitions
- DUCULA
- aenea
- palawana / palawanense / palawanensis / palawanorum / palawanus
The Key to Scientific Names
Legend Overview
Distribution
Northern Philippines (on Calayan, Fuga, and Camiguin Norte).
Identification Summary
This subspecies is like D. a. nuchalis, but larger (wing 265 mm, versus 226–237 mm), the nape and ear-coverts are chestnut, and the mantle has slightly stronger coppery reflections (29).
Ducula aenea fugaensis (Hachisuka, 1930)
Definitions
- DUCULA
- aenea
- fugaenis / fugaense / fugaensis
The Key to Scientific Names
Legend Overview
Distribution
Philippines (except the far north, and southern and southwestern islands).
Identification Summary
Similar to D. a. polia, but has the gray hindneck more sharply demarcated from the green mantle, the tail duller gray-green without any blue reflections, and the chin and throat darker, being more suffused with pinkish gray; and even more like D. a. palawanensis from which it differs solely by having a duller green tail. Wing 231–239 mm.
Ducula aenea aenea (Linnaeus, 1766)
Definitions
- DUCULA
- aenea
The Key to Scientific Names
Legend Overview
Distribution
Talaud Islands and Sangihe Islands, north of Sulawesi (Indonesia).
Identification Summary
Separated from the nominate subspecies by its dull metallic blue-and-green mantle with a gray wash (thus appearing less bright and iridescent), black undertail (not brown), and dusky-red legs and feet (not red). Compared to D. a. paulina, it lacks a rusty nape patch, has duller less metallic upperparts, dark mahogany (not dark chestnut) undertail-coverts, and a black underside to the tail, as well as being larger (wing 256–271 mm, versus 214–234 mm in D. a. paulina) (13).
Ducula aenea intermedia (Meyer & Wiglesworth, 1894)
Definitions
- DUCULA
- aenea
- intermedea / intermedia / intermedianus / intermedium / intermedius
The Key to Scientific Names
Legend Overview
Green Imperial-Pigeon (Maroon-naped) Ducula aenea nuchalis Scientific name definitions
Distribution
Northern Philippines (northern Luzon).
Identification Summary
Distinctive versus most other subspecies by its purplish-maroon or purplish-chestnut nape patch, as well as having much more heavily bronzed or coppery-glossed upperparts, and the tail is duller green (compared to D. a. polia, but like the nominate). Wing 226–237 mm. There be some introgression with the nominate (13).
Ducula aenea nuchalis (Cabanis, 1882)
Definitions
- DUCULA
- aenea
- nuchale / nuchalis
The Key to Scientific Names
Legend Overview
Green Imperial-Pigeon (Rufous-naped) Ducula aenea paulina Scientific name definitions
Distribution
Indonesia, on Sulawesi (and associated islands off the northeast coast), and the Togian Islands, Banggai Islands, and Sula Islands.
Identification Summary
Like D. a. nuchalis, has a prominent rusty nape patch (but larger, lighter, and much brighter than that of D. a. nuchalis) and rich green (strongly metallic and thus iridescent) upperparts , although these are not strongly demarcated from the gray hindneck, and has marginally darker underparts, especially on the foreneck. Wing 214–234 mm; those on the Sula Islands, sometimes separated as subspecies D. a. sulana, have wing 205–216 mm (13).
Ducula aenea paulina Bonaparte, 1854
Definitions
- DUCULA
- aenea
- paulina
The Key to Scientific Names
Legend Overview
Related Species
Relationships are not well-resolved; likely closely related to the formerly conspecific Enggano Imperial-Pigeon (Ducula oenothorax). Considered to form a species-group with Spectacled Imperial-Pigeon (Ducula perspicillata), Seram Imperial-Pigeon (Ducula neglecta), Elegant Imperial-Pigeon (Ducula concinna). In a molecular phylogeny of the genus Ducula, Ducula aenea was found to be sister to a clade that included Island Imperial-Pigeon (Ducula pistrinaria), Micronesian Imperial-Pigeon (Ducula oceanica), Pacific Imperial-Pigeon (Ducula pacifica), and Red-knobbed Imperial-Pigeon (Ducula rubricera) (30), although none of the taxa with which it is considered to form a species group with were included in the study.
Fossil History
Nothing known.
Distribution
Widespread across mainland South Asia, in western, southern, and eastern India, and Sri Lanka, east to southern China, Indochina, but absent from much of Thailand, and south again through the Thai-Malay Peninsula, thence through the Greater Sundas and Borneo, the Lesser Sundas east as far as Alor, Sulawesi, and north throughout the Philippines.
Extralimital Records
Possible sight record on Timor (Indonesia) (31).
Historical Changes to the Distribution
None definitely known, but there have perhaps been some local extinctions in Philippines and Indonesia (13).
Habitat
Forests, including both primary and secondary evergreen, and monsoon forests. Also coastal scrub, coconut groves (32), mixed gardens, mangroves (14, 28), nipah palm swamps (33), open country with scattered trees, savanna woodland, and second growth. Mainly found in the lowlands; in India reported up to c. 300 m, occasionally to 600 m (14) (in Sri Lanka breeds to 500 m 34); in Greater and Lesser Sundas reported to 1,000 m (28) (also on Taliabu, Sula Islands 35), or 1,050 m on Borneo (33), in Philippines to 1,125 m (36), and to 1,300 m on Hainan (southeast China) (37).
Migration Overview
Apparently resident, although nocturnal dispersal has been reported in Peninsular Malaysia (10). In southern Vietnam, where occurs mainly in mountains, only descends to lowland areas when these provide a rich food source. In Sri Lanka, reported to have regular roosting trees, sometimes located several km from feeding areas. Inter-island movements reported in some areas.
Feeding
Food Capture and Consumption
Often seen singly, in pairs, or small groups of up to five individuals (13), exceptionally of up to 50 or 60 birds (38, 39), regularly with other large pigeons, generally feeding in the upper canopy.
Diet
Major Food Items
Frugivorous, taking a large variety of fruits and berries, typically up to 40 mm in diameter (13), and on Sulawesi involving at least 33 species belonging to 14 families (40). Wild figs (Ficus) and nutmegs (Myristica) are reportedly favorite foods (15, 41, 10, 13); other recorded items include the buds of mangroves (Avicennia).
Drinking, Pellet-Casting, and Defecation
Drinking
At least in India, regularly descends to ground to drink water, or to ingest salt-rich soil (42).
Vocalizations
Vocal Array
A rather vocal species (43, 13). Most common vocalization is a low-pitched, growling or purring, bisyllabic rhu-rrhuuu with the emphasis on the final syllable and typically preceded (or followed) by a shorter and softer note (either can be variable in length, or absent), sometimes apparently given in duets (44, 43, 45) involving burry long notes being quickly followed by a much clearer one (14). One variation involves a similar-sounding rhythmic rhu-rhurhurhurhu, ending with a descending series of cooing notes. Also some geographical variation, e.g., in Indian Subcontinent region, very deep and growling on Andamans, where also highest at end (46), and noticeably higher-pitched overall in Sri Lanka (14), while a deep resounding wuck-wuck-woor or wuck-woor-woor-woor-woor that ends in laughing-like sound, and perhaps represents a more excited version of a typical advertising call, has also been heard in India (41); on Sulawesi, also gives a questioning woohoo woo, the first part upwardly inflected (47); and on Palawan (in the southwest Philippines), a similar deep-sounding ah-hoo-oo (48). Birds in mainland South-East have songs that in pitch are intermediate between those of Sri Lanka and the Andamans (14). Also single hoots, either with a sleepy growling or more pure tonal quality, lasting c. 1 second and repeated at intervals of c. 6 seconds.
Locomotion
Flight
Flight swift and powerful, usually well above the canopy.
Self-Maintenance
Roosting
In Sri Lanka, reported to have regular roosting trees, sometimes located several km from feeding areas.
Sexual Behavior
Courtship, Copulation, and Pair Bond
Male performs spectacular display-flight, commencing in normal flight, then suddenly beats wings vigorously and shoots vertically upwards over c. 2 m, before stalling momentarily with wings flexed and neck outstretched, as if about to tip over backwards, finally turning sharply and diving down, then resuming normal flight, or rises from trees to perform similar flight (44).
Social and Interspecific Behavior
Degree of Sociality
Often seen singly, in pairs, or small groups of up to five individuals (13), exceptionally of up to 50 or 60 birds (38, 39), regularly with other large pigeons.
Phenology
In India, breeds December–June (March–June in north, December–May in south, including Sri Lanka, and the Andaman Islands 14); in Peninsular Malaysia, it breeds in mid September and January–March (10); on Sumatra it breeds in most months, but mainly December–June (18); on West Java, it breeds in January (49); on Borneo, it breeds in August, December, and January (33); in the Lesser Sundas, it is said to be during April–July (26, 13), but on Flores, eggs were recorded in January, February, May, June, July, and October, and a well-feathered nestling in June, suggesting little seasonality, although there was a clear peak in May (50); and nests with eggs have been recorded on Negros (in the Philippines) in March–May (36, 51).
Nest Site
Site Characteristics
Nest is placed in a leafy sapling or understorey tree, usually less than 10 m up or 3–18 m above ground in mangrove forest on Tioman Island, off the east coast of Peninsular Malaysia (10), but also reported 25–30 m above ground in Philippines (36, 51).
Nest
Construction Process
Both sexes are involved in nestbuilding.
Structure and Composition
Eggs
Size
44.8–52.4 × 32.5–33.8 mm (D. a. polia, Flores 50).
Color and Surface Texture
Slightly glossy egg (50).
Clutch Size
Usually one white, rarely two.
Incubation
Incubation Period
Unknown.
Parental Behavior
Incubation by both sexes.
Population Status
The Green Imperial-Pigeon is widespread and remains common in much of its extensive range, e.g., in many parts of Sri Lanka, the Greater Sundas (28), Philippines (36), and northern Sulawesi, as well as some of its satellite archipelagos (32); also locally on Simeulue Island, off Sumatra (18). However, in India, the species is considered very local in both the Western Ghats and the South Assam Hills (14). It is generally the commonest Ducula within its Wallacean range, from Sulawesi and the Banggai and Sula Islands to the Lesser Sundas (52), despite effects of sometimes excessive hunting in some areas within this range, e.g., on Alor (53), and can survive in degraded habitats (54). Indeed, a study in Sabah (Malaysian Borneo) found that this species is increasing in logged forest (55), but on Java it is absent from most forest fragments (56). Apparently rare in other areas, e.g., mainland southeast China, where found at just two of 54 forested sites surveyed in 1997–2004 and is known only from southern Yunnan, southern Guangdong, and Hainan Island (57, 58); and on Bohol and Negros, in the east-central Philippines (59), in which archipelago it is probably extinct on Cebu and Panay (60). A major decline has been registered in Laos (61, 62); the species is reportedly extirpated in parts of Peninsular Malaysia due to excessive hunting and loss of plains-level forest (10); and numbers are probably much-reduced in Thailand due to dam construction in low-lying valleys (63). The distinctive subspecies D. a. intermedia might well have declined on Talaud (Indonesia) (64).
Conservation Status
Not globally threatened (Least Concern).
Management
Known from many protected areas throughout the species’ range, e.g., Rajah Sikatuna National Park, Bohol (Philippines) (65), Similajau National Park, Sarawak (66), Tanjung Puting National Park, Kalimantan (Indonesian Borneo) (67), and Manembonembo Nature Reserve, North Sulawesi (68).
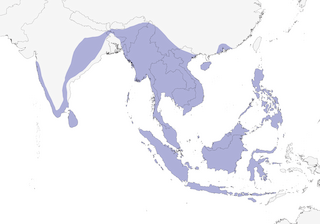
- Year-round
- Migration
- Breeding
- Non-Breeding

































