Gray Fantail Rhipidura albiscapa Scientific name definitions
Text last updated April 18, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | cua de ventall gris |
| Dutch | Grijze Waaierstaart |
| English | Gray Fantail |
| English (United States) | Gray Fantail |
| French | Rhipidure gris |
| French (France) | Rhipidure gris |
| German | Graufächerschwanz |
| Japanese | ハイイロオウギビタキ |
| Norwegian | gråviftestjert |
| Polish | wachlarzówka szara |
| Russian | Серая веерохвостка |
| Serbian | Siva lepezarka |
| Slovak | vejárnik sivý |
| Spanish | Abanico Gris |
| Spanish (Spain) | Abanico gris |
| Swedish | australisk solfjäderstjärt |
| Turkish | Gri Katmerkuyruk |
| Ukrainian | Віялохвістка сиза |
Rhipidura albiscapa Gould, 1840
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
The Key to Scientific Names
Legend Overview
Field Identification
14–17 cm; 6·5–9 g. Male nominate race has upperparts sooty grey, sides of face sooty, with short, narrow white supercilium and narrow white streak above ear-coverts; remiges sooty grey, wing-coverts with small white tips, inner secondaries with broad white edges; tail dusky grey-brown with narrow dull edging on outer web of rectrices; chin spot quite pale grey, upper breast with large, diffuse sooty-black band, lower breast and belly deep creamy-buff when fresh becoming dirty cream when worn, sides of breast deep grey, extending over upper flanks; iris dark brown; bill black, legs very dark brown. Female similar to male, but averaging smaller in wing and tail. Immature like adult but wing-coverts tipped pale rufous; juvenile duller with buff-rufous tipping to grey body feathers, buff suffusion through eyebrow, dull rufous tips to wing-coverts, throat white, pale dull rufous wash across breast, greyish at sides, rest of underparts with faint warm buffy-white glow. Racial variation marked and complex, mainly in Australia (where races vary primarily in depth of grey colour and in tail pattern): <em>alisteri</em> has upperparts lighter, side of face darker, breastband narrower (not extending across flanks), tail darker, rectrices with broader edging; <em>preissi</em> has upperparts mid-grey, side of face lighter, white tips of wing-coverts larger, tail greyer, chin spot absent, breastband small (not extending across flanks), remaining underparts lighter; albicauda is medium brownish-grey above, white wing-covert tips larger, chin spot absent, breastband small and lighter (not extending across flanks), remaining underparts pale, tail proportionally very long, central two feather pairs dusky grey-brown, rectrices 3–4 white with outer web marked with dark grey-brown, outer two pairs entirely white; <em>keasti</em> has upperparts, wing and tail dark grey-black, breastband very large and much blacker; pelzelni is lighter above, wing-coverts with almost no tipping, breastband small and poorly defined (not extending across flanks), remaining underparts greyer; bulgeri has tail mostly brown with conspicuous white feather shafts, outer rectrices edged and tipped white; brenchleyi is similar to previous, but underparts paler, shafts of central rectrices sometimes brownish.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Forms a clade with R. fuliginosa (1), and formerly considered conspecific, but differs markedly in vocalizations. See also R. phasiana. Races very similar, varying mainly in levels of saturation. Eight subspecies recognized.Subspecies
Gray Fantail (Melanesian) Rhipidura albiscapa [pelzelni Group]
Distribution
Rhipidura albiscapa bulgeri Layard, 1877
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- bulgeri
The Key to Scientific Names
Legend Overview
Distribution
Rhipidura albiscapa brenchleyi Sharpe, 1879
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- brenchleyi
The Key to Scientific Names
Legend Overview
Rhipidura albiscapa pelzelni Gray, 1862
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- pelzelni / pelzelnii
The Key to Scientific Names
Legend Overview
Gray Fantail (keasti) Rhipidura albiscapa keasti Scientific name definitions
Distribution
Rhipidura albiscapa keasti Ford, 1981
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- keasti
The Key to Scientific Names
Legend Overview
Gray Fantail (alisteri) Rhipidura albiscapa alisteri Scientific name definitions
Distribution
Rhipidura albiscapa alisteri Mathews, 1911
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- alisteri
The Key to Scientific Names
Legend Overview
Gray Fantail (albiscapa) Rhipidura albiscapa albiscapa Scientific name definitions
Distribution
Rhipidura albiscapa albiscapa Gould, 1840
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
The Key to Scientific Names
Legend Overview
Gray Fantail (preissi) Rhipidura albiscapa preissi Scientific name definitions
Distribution
Rhipidura albiscapa preissi Cabanis, 1851
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- preissi
The Key to Scientific Names
Legend Overview
Gray Fantail (albicauda) Rhipidura albiscapa albicauda Scientific name definitions
Distribution
Rhipidura albiscapa albicauda North, 1895
Definitions
- RHIPIDURA
- rhipidura / rhipidurus
- albiscapa
- albicauda
The Key to Scientific Names
Legend Overview
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Almost any wooded habitat, including parks and gardens, warm and cool rainforest, other forest and woodland (particularly edges and clearings), farmland with scattered trees, post-logging regeneration; also semi-arid scrublands, including mallee and acacia (Acacia) in Australia, riverine vegetation, exotic pine (Pinus) plantations, occasionally mangroves. Has adapted very well to European settlement and human-modified habitats. In Australia, mainly in wet and dry eucalypt (Eucalyptus) forests and woodlands, generally with tall trees, dense shrub layer and open subcanopy; on Norfolk I, present in most habitats with trees or shrubs from rainforest to gardens. In Australia, occurs from coast to 1500 m; at 500–900 m in Solomons (San Cristobal).
Movement
Migratory , nomadic or sedentary in different parts of range; movements may be related in some degree to seasonal conditions. In Australia, complex and poorly understood; some local populations augmented seasonally by immigrants from elsewhere. Those breeding in Tasmania and Bass Strait islands (nominate) winter in SE Australia, departing N in Mar–Apr, returning late Aug to Oct; part of population in SE & E Australia (alisteri) is resident, others migrate to N Australia and possibly New Guinea in autumn, returning Aug–Sept; in highlands of Atherton Tablelands and Clarke Range (keasti) mainly sedentary, with some altitudinal movement; C Australian birds (albicauda) resident or nomadic; in SW (preissi), some resident and some move to NW Australia. Possibly somewhat nomadic in non-breeding season in Vanuatu; movements in New Caledonia and the Solomons not known. In Australia, sometimes forms flocks of 20–200 individuals during migration. Longest known movement 1276 km in 38 months.
Diet and Foraging
Insects, usually flying ones; particularly Coleoptera (beetles), Diptera (flies) and Hymenoptera (bees, wasps, ants), all of which are taken by at least 50% of birds; less commonly consumed are Neuroptera (lacewings), Ephemeroptera (mayflies) and Hemiptera (bugs), which are taken by at least 20%. Also spiders, occasionally amphipods, lizards; occasionally fruit and seeds. In Australia, Jan–Mar, 72–82% of insects in stomachs were hymenopterans, mostly winged ants. Scans for prey by static searching from vantage point, less often by progressive searching. During static search, remains on perch for 5 seconds or more, with tail closed and wings slightly drooped, frequently turning around, but changing perch 7–9 times per minute on average; most prey, once located, captured in flight (73–100%), particularly by flycatching (71–86%), often 3–4 pursuit flights in quick succession; flights may be dives, ascents or sweeps, longest ones (more than 10 m) can involve 5–6 directional changes and last for 20 seconds; may capture more than one item on a sally. Less often, flying individual takes prey from surface of vegetation. When progressive searching, moves continually and rapidly through vegetation in short flights, hops and spins, sometimes using more than 20 perches per minute; wings drooped very low, tail widely spread. At times, gleaning may constitute 1–14% of captures. Feeds at all levels, from near ground to canopy, occasionally on ground; height varies seasonally, lower in winter, from higher perches when breeding. In Australia, considerable variation among studies owing to geographical and temporal differences: 0–24% on ground, 1–37% below 1 m, 13–52% at 1–5 m, 9–30% or more at 5–15 m, 1·7–25% above 15 m; in N, forages more aerially in dry season; where co-occurs with R. rufifrons, forages at higher level. Generally uses different perches for different feeding methods, mostly dead branches when static searching, primarily live twigs and branches when progressive searching. In Australia, foraging sites differ seasonally and regionally; from several studies, air 22–97% (in most more than 70%), ground 0·6–24% (in most less than 5%), among foliage 7–74% (in most 14–23%), on trunks of trees and saplings 1–14% (in most less than 5%), among twigs 0·6–5%, on branches c. 5%; in winter 86% air, 6% foliage, 3% branches, 1% trunk, 4% ground, in summer 77% air, 17% foliage, 5% branches, 1% trunk. Joins mixed feeding flocks comprising a range of small insectivorous species, including treecreepers (Cormobates), thornbills (Acanthiza) and robins (Petroicidae); most often in non-breeding season. Also follows domestic stock and humans.
Sounds and Vocal Behavior
Both sexes sing, female less than male; may sing on nest; prominent in dawn chorus in spring and early summer, starting 35–15 minutes before sunrise. Song, in Australia, a sustained chattering “tweet-a-tweet-a-tweeta”, becoming double warble to thin, squeaky, high-pitched, ascending series of whistles in seesaw cadence, sometimes likened to high notes of a violin; race albicauda a rapid trill-like series of short whistles of descending frequency. In Vanuatu and New Caledonia, short, high-pitched, often repeated series of short twittering notes, reminiscent of some Australian songs. Contact note a repeated single “tweet”; rapid piping notes as alarm.
Breeding
In Australia Aug–Jan, longer in S; nest-building and dependent young in Sept on San Cristobal (Solomons); Sept–Jan in Vanuatu; eggs in Sept and nests with young in Dec on Norfolk I; in Australia often 2 or more broods in a season, in Vanuatu usually 2 but sometimes 3. Solitary; territorial, disputes with rival birds occur along abutting territorial boundaries; mobs mammals and other perceived threats. Courtship consists of aerial chases, bouts of song and some perched displays; male feeds female during pair-formation and nest-building. Nest built by both sexes, in 2–20 days, a small cup, usually with “tail” hanging from base, in Australia external diameter 51–64 mm, depth 32–63 mm, internal diameter 38–44 mm, depth 25–32 mm, tail 25–178 mm, in Vanuatu external diameter 50 mm, external depth 30 mm, thickness of walls 5 mm; made from fine grass and shreds of bark, and sometimes moss, lichen, twigs or other plant material, thickly bound externally with spider web, sometimes with hair or wool, lined with fine grass, rootlets and hair, sometimes plant down, fuzz from fern fronds, bark fibre, moss or feathers; sites vary greatly, but usually placed on thin horizontal branch or fork, often sheltered by canopy overhead, sometimes overhanging water, and usually in tree, sapling or shrub but also in artificial structures, from below 1 m to 30 m up in Australia; defends territory of 1–2 ha. Nest abandonment before egg-laying is very common; pairs will build up to seven nests within a breeding season, and abandon up to 71% of their nests before egg-laying (4). Clutch in Australia 1–5 eggs, possibly 6, usually 3 (race keasti 2), mean 2·66; eggs white to pale yellowish-buff or yellowish-white, with light brown to reddish-brown, light umber or rufous speckles and spots (often with underlying markings of pale lavender or dull grey) forming cap or wreath around large end (sometimes scattered over whole shell), mean 15·8 × 12 mm in Australia, 16 × 12·5 mm on Norfolk I. Incubation usually from final egg, by both sexes, mean stint lengths c. 11·5 minutes, change-over at nest often 1–2 seconds (sitting bird sometimes allowing itself to be touched by observer), incubation period usually 14 days, sometimes 15 days, occasionally less; fledging period usually 10–15 days; young fed and attended by both parents for several weeks after leaving nest; new clutch often started while previous brood still being fed, female then incubates while male feeds fledglings. Frequently parasitized by cuckoos: in Australia, by Pallid (Heteroscenes pallidus), Fan-tailed (Cacomantis flabelliformis) and Brush Cuckoos (Cacomantis variolosus), Horsfield’s Bronze-cuckoo (Chalcites basalis) and Shining Bronze-Cuckoo (Chalcites lucidus); in Vanuatu, by Fan-tailed Cuckoo. In Australia, hatching success 41–57%, fledging c. 19% (41–59% of nests fledge at least one young); nest predators include Pied Currawong (Strepera graculina) in Australia. Both sexes can breed in first year. Longevity: an individual retrapped more than 9 years 8 months after first ringed.
Conservation Status
Not globally threatened. Common throughout range; may have increased owing to ability to adapt to human-modified habitats, although some apparent declines in highly urbanized areas. Widespread and common in Australia, especially in E & SW, but infrequent or absent in many desert areas; often killed by cars. Common and widespread on Norfolk I, but considered vulnerable owing to small size of island and limited population. On San Cristobal (S Solomons), moderately common at 500–900 m, unknown in lowlands.
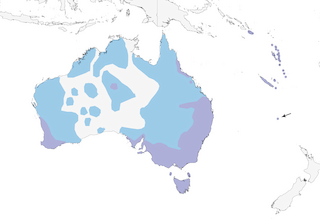
- Year-round
- Migration
- Breeding
- Non-Breeding
































