Gray-headed Fish-Eagle Icthyophaga ichthyaetus Scientific name definitions
- NT Near Threatened
- Names (26)
- Monotypic
Revision Notes
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Assamese | উকহ |
| Bulgarian | Сивоглав морски орел |
| Catalan | pigarg capgrís |
| Czech | orel šedohlavý |
| Dutch | Grote Rivierarend |
| English | Gray-headed Fish-Eagle |
| English (Kenya) | Gray-headed Fish Eagle |
| English (United States) | Gray-headed Fish-Eagle |
| Finnish | isokalakotka |
| French | Pygargue à tête grise |
| French (France) | Pygargue à tête grise |
| German | Graukopf-Seeadler |
| Indonesian | Elang-ikan kepala-kelabu |
| Japanese | ウオクイワシ |
| Malayalam | വലിയ മീൻപരുന്ത് |
| Norwegian | båndelveørn |
| Polish | rybożer białosterny |
| Russian | Сероголовый рыболов |
| Serbian | Sivi belorepan |
| Slovak | orliak rybár |
| Spanish | Pigarguillo Común |
| Spanish (Spain) | Pigarguillo común |
| Swedish | gråhuvad fiskörn |
| Thai | เหยี่ยวปลาใหญ่หัวเทา |
| Turkish | Balıkçı Kartal |
| Ukrainian | Орлан-рибалка великий |
Revision Notes
Luca Bielski prepared the account for the 2023 Clements taxonomy update.
Icthyophaga ichthyaetus (Horsfield, 1821)
Definitions
- Icthyophaga
- ICHTHYAETUS
- ichthyaetus
The Key to Scientific Names
Legend Overview
Field Identification
61–75 cm; 1,500–1,600 g in males, and 2,300–2,700 g in females (1); wingspan 155–170 cm. Medium-sized brownish-gray fishing-eagle, with relatively small head and bill gray, and belly and base of tail white; iris yellow, with cere gray-brown to blackish-gray, and legs grayish-white to pale yellowish-gray (2). Female is usually larger than male by up to 23%, and apparently much heavier (2, 1). Juvenile is brown, with underparts usually heavily streaked white (one specimen from Java was unstreaked); iris brown (not yellow).
Similar Species Summary
Darker and duskier than Lesser Fish-Eagle (Icthyophaga humilis), which is more associated with foothills and faster-flowing waters, and has poorly marked subterminal tailband and small white flashes at base of primaries (2). Pallas's Fish-Eagle (Haliaeetus leucoryphus) is larger, with significantly longer but narrower wings, paler head, and dark abdomen, thighs and undertail coverts (2).
Similar Species
Plumages
Gray-headed Fish-Eagle has 10 full-length primaries (numbered distally, from innermost p1 to outermost p10), 16–17 secondaries (numbered proximally from outermost s1 to innermost s13 or s14, and including three tertials, numbered distally t1–t3), and 12 rectrices (numbered distally, r1–r6, on each side of the tail). Accipitrid hawks including Gray-headed Fish-Eagle are diastataxic (see 3), indicating that, through evolution, a secondary has been lost between what we now term s4 and s5. Little or no geographic variation in plumages have been reported. The following is based on descriptions in Ali and Ripley (4), Thiollay (5), Wells (6), and Naoroji (7), along with examination of Macaulay Library images. See Molts for molt and plumage terminology. Sexes are similar in all plumages; definitive appearance is attained at the Third or Fourth Basic Plumage. Timing of plumages generally may revolve around a peak breeding season of December-March in the southern parts of the range, but fresh and worn examples of all plumages likely can be found year-round among other populations (see Breeding).
Natal Down
There is little published information on natal down of Gray-headed Fish-Eagle but Macaulay images (see below and ML374255471 ) indicates that it may be rufous, whitish, and buff in coloration.
Juvenile Plumage
Juvenile Plumage is mostly brown with white streaking. The head, neck, and breast feathers have pale shafts and subterminal tips resulting in a streaked appearance. Upperwing coverts are dark brown with pale tips. Primaries and secondaries are narrow, tapered at the tips, even in wear, blackish brown above, and buff with dark brown barring below. Underparts can appear buff with dark streaks or dusky with pale streaks. Underwing coverts are mostly whitish to buff with dark tips to some lesser and median coverts, sometimes forming a thin dark band. Rectrices are narrow, tapered at the tips, and dirty brown from above and pale from below, with a dark brown subterminal band.
Formative Plumage
Examination of Macaulay Library images indicates that this species undergoes a limited Preformative Molt (cf. 8). Formative Plumage is similar to worn Juvenile Plumage except new, dark formative feathers are present on the hindneck and mantle, and among the scapulars. Some breast feathers may also be replaced and are fresher and more solidly darker brown than juvenile feathers, or tinged rufous. Retained juvenile head and flight feathers become worn and abraded.
Second and Third Basic Plumages
Second and Third Basic Plumages represent variable transitional stages between Juvenile and Definitive Basic Plumages. The head and neck become grayer but retain pale and dark streaking. The upperparts are mottled brown and gray-brown. The bases of the rectrices become white with decreasing dusky mottling, and the white tips to the subterminal tail band becomes less extensive or absent. Second basic inner primaries and secondaries are partially barred, more so for feathers replaced earlier in molt, such as p1–p6, s1, s5–s6, secondaries near the tertials. The breast and underwing coverts become darker brown, with less pale streaking or mottling.
Examination of Macaulay Library images indicates that this species undergoes Staffelmauser (see Definitive Basic Plumage and Molts). The Second Prebasic Molt appears often to be complete but at least occasionally up to one or more juvenile outer primaries and some juvenile secondaries (most likely among s3–s4 and s8–s11) can be retained, narrow, brown, and worn, and highly barred, contrasting with replcaed darker and less-barred feathers. Third Basic Plumage can be recognized by ongoing complete or incomplete molts but with retained barred second basic primaries and secondaries, especially, e.g., those among p5–p7 and earlier replaced secondaries. Body plumage is more definitive-like but can retain some brown in the head, pale streaking to the underparts, and pale brown and white mottling to the underwing coverts. Study is needed on precise age determination in Gray-headed Fish-Eagle; it may be possible that some birds in Fourth Basic Plumage can be recognized by Stafflemauser molt patterns along with a few retained, slightly barred primaries and secondaries, and perhaps also underwing coverts that have not become fully dark brown.
Definitive Basic Plumage
Head and neck are grayish, the center crown is washed brown, transitioning to brownish gray back, and a dark brown lower upperparts and breast, the latter sometimes tinged rufous. Rectrices are broader and more truncated at the tips than juvenile rectrcies, white at the base with a broad and distinct dark brown terminal band. Upperwing coverts, remiges, and underwing coverts are uniformly dark brown (although mottled paler brown when some feathers become worn or during molt). The lower underparts and undertail coverts are white, often with sparse dark streaking or barring to the femoral feathers. Molt of remiges can be complete but following incomplete molts, mixed generations and levels of wear occur, showing 2‒4 sets of basic feathers in Staffelmauser (or stepwise) patterns (see Molts and 9, 10).
Molts
General
Molt and plumage terminology follows Humphrey and Parkes (11), as modified by Howell et al. (12; see also 13 , 14, and 15 ). Under this nomenclature, terminology is based on evolution of molts along ancestral lineages of birds from ecdysis (molts) of reptiles, rather than on molts relative to location or time of the year (see 10,16 for more information). Little information has been published on molts in this species but based on examination of Macaulay Library images, Gray-headed Fish-Eagle appears to exhibit a Complex Basic Strategy (cf. 12, 17), including incomplete to compete prebasic molts and a limited preformative molt but no prealternate molts, as common in Accipitriformes. Remigial replacement undergoes Staffelmauser (or stepwise) molting patterns. In southern portions of the range, timing of molts may generally follow the peak breeding season of March-November but replacement of flight feathers appears to be protracted and likely can be found throughout much of the year within individuals and year-round at the population level (cf. 6).
Prejuvenile (First Prebasic) Molt
Complete, in the nest. No detailed information on this molt in Gray-headed Fish-Eagle.
Preformative Molt
Examination of Macaulay Library images indicates that a Preformative Molt occurs as in other Accipitriformes (8), and is limited, including a few to some or most hindnape, back feathers, and scapulars, with scattered feathers to the underparts as well (see images under Formative Plumage). It may occasionally be absent. Molt likely occurs from 4 to 10 months following fledging.
Second and Third Prebasic Molts
Examination of Macaulay Library images indicates that prebasic molts can often be complete but are sometimes incomplete. Sequence of flight-feather replacement as in the Definitive Prebasic Molt (below). Following the Second Prebasic Molt, up to one or more juvenile outer primaries and corresponding primary coverts, and 3–4 or more juvenile secondaries (typically among s3–s4 and s8–s11) may often be retained. Staffelmauser replacement patterns (18, 9, 10) ensue (see below), the Third Prebasic Molt commencing where Second Prebasic Molt arrested and new sequences commencing at p1, the tertials and secondaries (see images under Second and Third Basic Plumages). In slower-molting individuals juvenile secondaries (s4 and s9–s11) may be retained during the Third Prebasic Molt and barred second basic feathers likely also can retained for two years, allowing identification of Fourth Basic Plumage (study needed). During these initial remigial molts, symmetry is usually maintained between the wings but patterns can begin to become asymmetrical following the Third Prebasic Molt.
Definitive Prebasic Molt
Sometimes complete but often incomplete, especially in for Definitive Prebasic Molts in adults, for which timing for molt is constrained by breeding activities. A rather high proportion of Macaulay Library images show birds replacing primaries, indicating that molt may be protracted, occurring rather continuously (cf. 6) but likely peaking from 1 to 5 months following breeding and perhaps also sometimes suspended during incubation and continued after fledging as can occur in other raptors (14). Most to many body feathers, upperwing coverts, and rectrices are usually replaced every year, but some feathers may be retained; remiges are often (but not always) partially replaced every year. Primaries are replaced distally (p1 to p10), secondaries are replaced proximally from s1 and s5 and distally from the tertials (or likely bilaterally for the second tertials, t2), and rectrices may generally be replaced from the central feathers (r1) distally on each side of the tail, although the outer rectrix appears to be molted before feathers among r3-r5 as is common in Accipitridae.
Molting patterns among primaries and secondaries exhibit Staffelmauser (18, 14, 9, 10) whereby incomplete molts result is a series of commencement points, beginning with termination points of previous prebasic molt and also initiating new series commencing at p1, the tertials, s1, and s5. Replacement thus typically proceeds in 2–4 waves through the wing. After several years, replacement patterns can become quite asymmetrical between the wings. Staffelmauser appears to be a product of insufficient time to undergo a complete wing-feather molt but has adaptive benefits in producing multiple small gaps in the wing during molt, which retains wing integrity and ability to fly and forage (19, 20, 14).
Bare Parts
The following is based on Ali and Ripley (4), Thiollay (5), and Wells (6), along with examination of Macaulay Library images to evaluate changes in bare-part coloration with age. See also images under Plumages.
Bill
The bill is large, relatively long, and powerful, with a deep hook at the tip. At all ages, the cere and bill are primarily dark slate to blackish, the lower mandible often being paler or mottled slate blue, especially at the base.
Iris and Facial Skin
In adults, the iris is typically pale yellow. In juveniles it can be brownish to dark hazel. It appears to gradually become more yellowish through the first, second, and third cycles, perhaps taking four or more years to become fully pale yellow. The orbital skin is gray.
Tarsi and Toes
The legs and feet are large, powerful and scaly, with spiny soles and strong, curved talons. In adults, they are pale yellow or tinged olive with blackish claws. In younger birds they are duller and grayer, becoming yellower during the first year.
Measurements
Mass
1,500–1,600 g in males, and 2,300–2,700 g in females (1).
Systematics History
Population of Sri Lanka sometimes awarded separate subspecies, plumbeiceps, differing only in slightly smaller size; not normally accepted.
Subspecies
Distribution
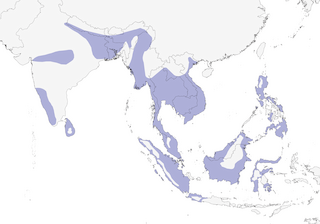
India, Nepal, and Sri Lanka east through Indochina and the Malay Peninsula to the Greater Sundas, Philippines, and Sulawesi.
Habitat
Lowland forests, mainly below 300 m, occasionally to ca. 1,500 m (2); closely associated with large bodies of fresh water, particularly sluggish streams and large ponds. Found also at rivers, reservoirs (21), and marshes, and in Cambodia locally at seasonally flooded swamp-forest (22); occurs also at mangrove swamps (21) and along sea coasts, sometimes near estuaries.
Movement
Sedentary.
Diet and Foraging
Almost exclusively fish; probably also some terrestrial birds (e.g., Gallus junglefowl) (2) and small mammals (e.g., squirrels) (2), rarely reptiles. Fish taken include introduced exotic species, including large-bodied catfish (21). In Singapore, observations during 2009–2011 at three widely separated sites identified prey as consisting of at least five fish species and one reptile, four of the fish being non-native species, i.e., two species of Pangasius catfish (both possibly introduced for human food), the Neotropical Cichla orinocensis (for sport-fishing) and the Amazonian Geophagus altifrons (pet trade); the other fish species, i.e., striped snakehead (Channa striatus), and the reptile, a water monitor (Varanus salvator), are both native species (21). Catches fish from water surface in short flight from hunting perch, but will also search for prey during quartering flight (2); fish too large to carry may be dragged to bank. Will also eat dead fish.
Sounds and Vocal Behavior
Often vocalizes at night, but only during breeding season; most frequently heard is a loud clanging which is far-carrying and resembles the voice of Indian Gray Hornbill (Ocyceros birostris); also a high-pitched scream, an owl-like ooo-wok , and loud gurgling calls (2).
Breeding
Little studied. Season December–March in Sri Lanka, November–January in India; nests found in January and March in Myanmar, April in Sumatra, probably August in Borneo (2); in seasonally flooded swamp-forest at north Tonle Sap, in Cambodia, breeding cycle starts when floodwater begins to rise, in September, and ends when waters fully receded, in March (22). Adults quite vocal during courtship. Builds huge stick nest 8–30 m (2) up in tall tree in forest, near lake or sluggish stream. In seasonally flooded swamp-forest in Cambodia, selected relatively tall trees with open crown structure, preferring trees closer to permanent water than expected (but timing of breeding not affected by distance to permanent water), preference for proximity to water perhaps reflecting some advantage in terms of prey availability, while human habitation negatively correlated with rates of nest-site occupancy (22); nests used for several years in succession. Clutch usually 1–2 eggs, occasionally up to 4 (2); incubation by both sexes, period 28–30 days; fledging period ca. 10 weeks; no information on fledgling dependency period.
Conservation Status
Not globally threatened. Currently considered Near Threatened. CITES II. Widespread, but only locally common, and population possibly moderately small and believed to be suffering fairly rapid decline. Little information on numbers, but BirdLife International suggests global total of fewer than 100,000 mature adults (fewer than 150,000 individuals in all). Seems to be widespread and locally frequent in northeastern India, but scarce and local in peninsula; rare and local in Nepal and in Sri Lanka, widespread but local and uncommon in Bangladesh, scarce or rare in Myanmar, rare and apparently declining in Philippines (formerly quite common in north and east), rare and confined to south in Thailand (formerly widespread), rare in Laos, scarce in southern Vietnam (and fast disappearing from north of that country), scarce and declining in Cambodia, uncommon and sparse with perhaps 40 pairs remaining in Peninsular Malaysia (previously common), widely distributed but uncommon in Sumatra and Borneo, very rare in Java, and rare and local in Sulawesi. Rare on Buton (off southeastern Sulawesi), where first recorded during surveys in Lambusango Forest Reserve during June–August in 1999, 2001–2003, 2005 and 2008–2010 (23). Scarce in Singapore, but probably increasing there (at least temporarily) owing to its ability to hunt in waterbodies near urban areas and tolerate heavily degraded habitats, and its easy access to possibly abundant alien species (21). Once thought to be fairly numerous in many areas, e.g., in southern Sumatra was common in reserve of Padang Sugihan in mid-1980s and in northern Lampung in 1990s (24). Main causes of decline are loss of forested wetlands, overfishing, siltation, pollution and persecution. May compete with humans for food, e.g., those breeding in seasonally flooded swamp-forest at northern Tonle Sap are at least partly dependent on water-snakes as prey and may be threatened by unsustainable harvesting of these by local human population; may be threatened also by construction of hydropower dams upstream in China, Laos, Thailand and Cambodia, which could drastically alter Lake Tonle Sap flood cycle (22). Proposed conservation measures include surveys to reveal important areas, combined with regular monitoring at various sites throughout range; forests in areas known to be important to this species should be protected. In addition, public awareness campaigns should be undertaken to help local people to feel pride for the species and to encourage them to take better care of wetland habitats.
About the Author(s)
Chuenchom Hansasuta received a Doctor of Dental Surgery from Chulalongkorn University. During her long and distinguished career in dentistry, she studied and practiced in places such as Thammasat University (Thailand), State University of New York at Buffalo (USA), and University of Connecticut Health Center (USA), retiring in 2020. Chuenchom always had an intense curiosity for birds that evolved over time into an acute interest in plumages. She has long been active in education and volunteering, becoming chairwoman of The Flyway Foundation and actively engaging and educating the public in the study of birds and their plumages.
- Year-round
- Migration
- Breeding
- Non-Breeding






























