Light-mantled Albatross Phoebetria palpebrata Scientific name definitions
- NT Near Threatened
- Names (28)
- Monotypic
Text last updated March 31, 2014
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Afrikaans | Swartkopalbatros |
| Bulgarian | Пепеляв албатрос |
| Catalan | albatros de mantell clar |
| Czech | albatros světlehřbetý |
| Dutch | Roetkopalbatros |
| English | Light-mantled Albatross |
| English (Australia) | Light-mantled Sooty Albatross |
| English (New Zealand) | Light-mantled Sooty Albatross |
| English (United States) | Light-mantled Albatross |
| French | Albatros fuligineux |
| French (France) | Albatros fuligineux |
| German | Graumantelalbatros |
| Icelandic | Grátrosi |
| Japanese | ハイイロアホウドリ |
| Norwegian | gråalbatross |
| Polish | albatros ciemnogłowy |
| Portuguese (Brazil) | piau-de-costas-claras |
| Portuguese (Portugal) | Piau-de-dorso-claro |
| Russian | Светлоспинный альбатрос |
| Serbian | Beloleđi čađavi albatros |
| Slovak | albatros sivochrbtý |
| Spanish | Albatros Tiznado |
| Spanish (Argentina) | Albatros Manto Claro |
| Spanish (Chile) | Albatros de manto claro |
| Spanish (Spain) | Albatros tiznado |
| Swedish | ljusryggig albatross |
| Turkish | Gri Sırtlı Albatros |
| Ukrainian | Альбатрос довгохвостий |
Phoebetria palpebrata (Forster, 1785)
Definitions
- PHOEBETRIA
- palpebrata
The Key to Scientific Names
Legend Overview
Introduction
The Light-mantled Albatross is a striking, dark albatross of the Southern Ocean. It was formerly considered conspecific with the Sooty Albatross (P. fusca), the only other species in the genus Phoebetria. Like that species, it is dark brown overall, but Light-mantled is readily distinguished by its namesake, pale brown mantle. Differences in courtship display and timing of breeding prevent interbreeding in locations where the two co-occur. Light-mantled breeds from South Georgia Island east to Campbell and Antipodes islands, nesting biennially alone or in small colonies. In the non-breeding season, it disperses through the Southern Ocean north to central Chile and Argentina.
Field Identification
78–93 cm (1); male 2800–3360 g, female 2620–3700 g (2); wingspan 183–232 cm (1). Long, narrow, pointed wings and long, wedge-shaped tail distinguish present genus from Diomedea. Adult has sharply contrasting white crescent framing upper half of eye, connecting at rear with similar but shorter crescent framing lower half hardly to midpoint, where terminus is square-cut , otherwise entirely sooty-brown, darkest on head and becoming paler and greyer on body , being palest (ash-grey) on mantle, back and breast, less so on rump and uppertail-coverts; scapulars and upperwing sooty-brown or sooty-grey, paler than face but clearly contrasting with pale mantle and back; iris dark brown; bill black with narrow pale bluish sulcus (central line along ramicorn) not reaching bill tip and less obvious distally; legs pale pink-flesh or flesh-grey. From above, looks obviously dark-headed with large pale saddle reaching back , unlike P. fusca, but worn adult of latter can be tricky; at close range following are useful: when at rest, crown not rounded but peaks just behind eye ; culmen profile slightly more concave and colour of sulcus usually helpful, the white partial eyering abruptly ends rather than tapers away below central eye; fundamentally allopatric Phoebastria nigripes also almost all dark, but shows at least some white at bill base, and has shorter, more square-ended rather than wedge-shaped, tail, the feet projecting beyond it. Sexes alike, but male larger in most measurements (e.g. wing 503–552 mm, versus 490–526 mm in female) (2). Juvenile much like adult but sulcus darker and duller (brownish to grey) (1), as are shafts to flight feathers, and partial eyering is also dirtier.
Systematics History
Subspecies
Distribution
Circumpolar in Southern Ocean, breeding from South Georgia E to Campbell I and Antipodes Is.
Habitat
Marine and pelagic , generally in much colder waters than P. fusca. Breeds on remote oceanic islands , occupying steep slopes or cliff ledges, often inland and amongst vegetation , especially tussock grass or ferns.
Movement
Disperses widely over Southern Ocean, ranging from pack ice N to c. 33° S, reaching c. 20° S in zone of Humboldt Current, exceptionally N to C California in mid-Jul 1994 (1); generally driven N in winter by expansion of pack ice; has been observed as far S as 77º 50’ S (2). Rare off the Falklands (3), despite breeding as close as South Georgia, but has wandered N in Atlantic to Bahia (Brazil), at 12º S, in Jul–Aug (4). Normally keeps further S than P. fusca, especially younger individuals, with breeding birds typically foraging S of their colonies, those on Macquarie I travelling 1700 km to Antarctic waters (5).
Diet and Foraging
Mainly cephalopods (e.g. Moroteuthis ingens (6), Psychroteuthis glacialis, Kondakovia longimana, Teuthowenia, Histioteuthis) and Antarctic krill (Euphausia superba); also takes other crustaceans, fish and carrion, including remains of birds found floating at sea (e.g. Pachyptila prions), scraps of penguin skin, viscera and sea mammal blubber (6). Fish include Magnisudis prionosa, Electrona carlsbergi, Gymnoscopelus sp., Melanonus gracilis and Melamphaes sp., while squid and octopus number at least 46 species from 15 families (Lycoteuthidae, Enoploteuthidae, Octopoteuthidae, Onychoteuthidae, Gonatidae, Histioteuthidae, Psychroteuthidae, Architeuthidae, Neoteuthidae, Ommastrephidae, Lepidoteuthidae, Batoteuthidae, Chiroteuthidae, Mastigoteuthidae, Cycloteuthidae, Cranchiidae, Alloposidae), and crustaceans include Gnathophausia gigas, Cyllopus lucasii, Themisto gaudichaudii, Eurthyenes sp., E. obsesus, Euphausia superba, E. vallentini, Pasiphaea longispina, Acanthephyra sp. and Austropandalus grayi (5). At colonies on Crozets, squid accounted for 56·4% of diet (by wet mass), crusacea formed 16% (but dominated diet numerically) and fish 10·9%; in this region Antarctic krill was the most important crustacean, while in other areas this species may be the exclusive prey (6). Other data on regional dietary preferences reveal that those birds breeding on Macquarie I have greater similarities to those on Marion I than to those on the Crozets and South Georgia, due to the greater importance of fish and reduced importance of crustaceans at Macquarie; squid species taken were similar at Macquarie and Heard, with a greater proportion of Histioteuthis eltaninae and Martialia hyadesi at the first-named, compensated by greater consumption of Galiteuthis/Teuthowenia spp. at Heard, while species composition at Macquarie differed less in 1970–1993 than between seasons, either due to real stability in diet or to chance sampling during years of similar diet, prey availability or abundance (7). Very large squid have been recorded being taken by this species, including a 5·3 kg Kondakovia longimana (6). Catches prey by surface seizing (78% in one study) (6), but also by diving or surface plunging (7%) (6) and filtration (for krill, 15%) (6). In diving attains maximum depth of 12 m, regularly to c. 5 m (8). Perhaps mainly nocturnal feeder. Usually feeds alone, but sometimes in company of D. exulans; also known to associate with cetaceans, albeit perhaps not as readily as some other albatrosses (9) and around South Georgia this species appears shun mixed-species feeding frenzies (10). Occasionally follows ships and attends fishing boats. Brown Skua (Catharacta antarctica) has been observed attempting to kleptoparasitize this species in Indian Ocean (11).
Sounds and Vocal Behavior
Main vocalization is bisyllabic Sky-call given on or close to nest-site, with head and bill held vertically (2). Uses bill-snaps to intimidate conspecifics (2).
Breeding
Biennial, when successful, with breeding achieved in following season by 59% of failed pairs (2); starts Sept/Oct (e.g. arrives first week of Oct on Macquarie I) (12), with 10–12-day laying period typically commencing in late Oct, and fledging usually occurs in late May/early Jun (2). Often solitary or very loosely colonial in colonies of up to 12 pairs (2); low, conical nest of mud and vegetation, up to 20 cm high and 30–40 cm across with central depression, often protected by rock wall on one side (2). Single white egg , size 98–107 mm × 63–70 mm, mass 210–280 g (5); incubation c. 67–70 days (2) with stints of 4–24 days (mean 11·5 days, excluding first and last shifts) (2); chick has greyish down, whitish on face and is very similar to that of P. fusca (13), brooded for first c. 20 days and fed every 2·1–2·9 days (2); fledging c. 141–170 days. Sexual maturity at 7–12 years, but first returns to colony when six years old (5). Overall, each pair probably breeds successfully only once every 3–4 years, with mean breeding success just 35% (2). Adult survival c. 97·3% (2).
Conservation Status
Not globally threatened. Currently considered Near Threatened. Australian populations ranked as Endangered on national list (14). In late 1980s, world population was tentatively estimated at c. 30,000 breeding pairs and c. 150,000 birds; 5000–10,000 pairs in New Zealand area, 8000 pairs at South Georgia , 3000–5000 pairs at Kerguelen Is, and 2280 pairs at Crozet Is; more recently BirdLife International mentions c. 1949 pairs in Crozets, 1250 pairs on Macquarie I, 5000–7500 pairs on South Georgia (15), 3000–5000 pairs on Kerguelen Is, c. 5000 pairs on Auckland Is , at least 1600 pairs on Campbell I, 170 pairs on Antipodes Is, 200–500 pairs on Heard I, 350 pairs on Marion I and 129 pairs on Prince Edward I, equating to total annual breeding population of c. 19,000–24,000 pairs, or 58,000 mature individuals (and 87,000 individuals in total). Population trends poorly known: on Possession I (Crozets), decline of 1·7%/year between 1966 and 1995 (16), but now increasing (916 pairs in 2006) (17), while small population on Marion I now appears stable, following decrease in 1997–2002 (18) and may even be increasing (200 pairs in 1989, 350 pairs in 2007), and that on Prince Edward I has seen small increase in period 2002–2009 (92 (19)–129 pairs). At-sea abundance in some areas has declined dramatically, e.g. in Prydz Bay region of E Antarctica since 1980/81 (20). However, majority of colonies not studied, though species probably declining owing to bycatch on longline fisheries (data are considerably sparser for this species compared to other albatrosses, but it has been taken by Japanese longliners in Australian waters) (21), plus perhaps impacts of introduced predators at some sites (e.g. cats on Kerguelen Is); a moderately rapid population decline is precautionarily suspected to be taking place over 100 years. Effects of global warming unlikely to be positive for this species, unlike P. fusca, as in Indian Ocean species’ breeding performance is apparently poorer in years with higher than normal sea-surface temperatures (22). Formerly exploited for food at some breeding stations, e.g. Kerguelen and Prince Edward Is. Main causes of breeding failure at South Georgia and Crozet Is were starvation of chicks and desertion by parents.
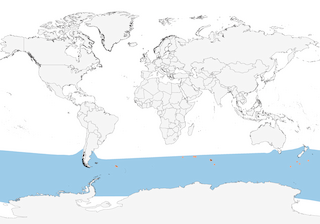
- Year-round
- Migration
- Breeding
- Non-Breeding
































