Red-billed Scythebill Campylorhamphus trochilirostris Scientific name definitions
Text last updated December 5, 2012
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | bec de dalla bec-roig |
| Dutch | Kolibriemuisspecht |
| English | Red-billed Scythebill |
| English (United States) | Red-billed Scythebill |
| French | Grimpar à bec rouge |
| French (France) | Grimpar à bec rouge |
| German | Rotrücken-Sensenschnabel |
| Japanese | アカユミハシオニキバシリ |
| Norwegian | rødbuetreløper |
| Polish | drzewiarz czerwonodzioby |
| Portuguese (Brazil) | arapaçu-beija-flor |
| Portuguese (Portugal) | Arapaçu-beija-flor |
| Russian | Красноклювый дугоклюв |
| Serbian | Crvenokljuna srpokljunka |
| Slovak | klzáčik červenozobý |
| Spanish | Picoguadaña Piquirrojo |
| Spanish (Argentina) | Picapalo Colorado |
| Spanish (Ecuador) | Picoguadaña Piquirrojo |
| Spanish (Panama) | Picoguadaña Piquirrojo |
| Spanish (Paraguay) | Picapalo colorado |
| Spanish (Peru) | Pico-Guadaña de Pico Rojo |
| Spanish (Spain) | Picoguadaña piquirrojo |
| Spanish (Venezuela) | Trepador Pico de Garfio |
| Swedish | rödnäbbad skärnäbb |
| Turkish | Kırmızı Gagalı Tırpangaga |
| Ukrainian | Дереволаз-серподзьоб середній |
Campylorhamphus trochilirostris (Lichtenstein, 1820)
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
The Key to Scientific Names
Legend Overview
Introduction
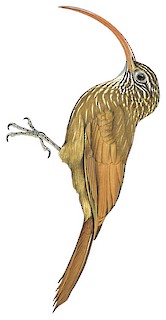
Red-billed Scythebill is a widespread woodcreeper of southern Central America and the lowlands of South America. This beautiful songbird is found from Panama south through much of northern and central South America in the under- and midstory of a variety of woodland types, typically below 1000 meters in elevation. This species is highly distinctive in most of its range, where it can be identified by its exceedingly long, decurved, red bill, and generally rusty olive plumage, with buffy streaking on the head, upper back, and chest. Red-billed Scythebill feeds on arboreal invertebrates by hitching along trunks and limbs, and often accompanies mixed flocks of other songbirds.
Field Identification
22–28 cm; 30–55 g. Slim, medium-sized woodcreeper with very long, slim, strongly decurved bill . Nominate race has finely streaked face and neck, weakly defined supercilium; brownish-olive crown and nape darker than reddish olive-brown back, crown with elongate whitish to ochraceous spots narrowly edged blackish, these becoming narrower and more elongate on nape, and reduced to fine shaft streaks on upper back; brown of back blending into cinnamon-rufous rump; remiges rufous-chestnut, tail slightly darker, wing coverts heavily edged reddish olive-brown, secondaries lightly edged with similar colour, tips of primaries dusky; chin and throat white, throat with fine brownish streaks; underparts brown, slightly lighter than above, breast heavily streaked with buff (lacking dark margins), streaks becoming narrower and shorter on belly and disappearing on lower belly and undertail-coverts; underwing-coverts light cinnamon to ochraceous; iris dark brown to hazel; bill bright red or reddish-brown, often dusky at tip and towards base; legs and feet greyish-olive to dull pea-green. Differs from nominate group of C. procurvoides in weaker contrast between back and rump, more extensive streaking on upper back, streaks above and below broader to tip (not narrowing to fine arrow-like point), legs slightly longer, bill paler, redder and less strongly curved; even more similar to “multostriatus group” of C. procurvoides, which is also heavily streaked above, but remaining characters still separate the species, and bill of multostriatus is dark-brown to reddish-chestnut; C. falcularius is darker overall, with back more extensively olive and rufous limited to uppertail coverts, streaking below narrower and more limited, crown and bill blackish; C. pusillus is also darker and more olive overall, more narrowly streaked above and below, with crown blackish, and bill brownish (less reddish) and shorter. Female is slightly smaller than male. Juvenile resembles adult, but plumage less richly coloured, streaking less clearly defined and more ochraceous, bill markedly shorter and darker (dusky blackish basally, reddish-brown distally). Within “trochilirostris group”, race <em>lafresnayanus</em> is larger and especially longer-billed then nominate, has underparts bright tawny-ochraceous, back brighter rufous (almost same color as rump, wings, tail); hellmayri is larger still, with longer and more robust bill; major is most similar to lafresnayanus but links it with nominate morphologically (and geographically) in having upperparts less rufous and bill much shorter; size and bill length similar to nominate, but overall coloration slightly paler, wings and tail more cinnamon or rufous, lower throat may be more boldly streaked. Within Amazonia, race devius is similar to lafresnayanus but with bill much shorter, plumage decidedly darker, and lower throat strongly washed with buff; snethlageae is similar to devius but underparts darker and with rufescent tinge, streaks on crown narrower, and throat whiter; notabilis is similar to snethlageae but overall coloration paler brown, and streaking above and below both broader and whiter. Despite its Amazonian distribution, race napensis is most similar to thoracicus but bill more strongly decurved, crown browner (less black), dark borders of streaks narrower and less conspicuous. Members of “venezuelensis group” are longer-billed than nominate, also darker overall, throat buffier and streaked more extensively and more heavily, crown blackish instead of brown, back and underparts darker olive-brown, and rump, wings and tail deeper rufous-chestnut; race brevipennis is darker and slightly smaller than venezuelensis , but has a proportionately longer bill. Birds of “thoracicus group” are similar to venezuelensis in coloration but streaks above and below have distinct, black borders; zarumillanus is said to be larger than thoracicus, with bill longer, more weakly curved.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Suggested as sister-species of C. procurvoides; also close to C. falcularius (which see), and sometimes considered conspecific. Assessment of geographical variation and separation from C. procurvoides both complicated by marked individual variation in general coloration and in extent and width of streaking above and below, but at least three geographically isolated groups evident: “venezuelensis group” in E Panama and N South America; “thoracicus group” along Pacific coast of South America; and “trochilirostris group” from Amazonia, Atlantic Forest and dry interior of South America. In last-mentioned group, affinities of Amazonian races problematic; napensis morphologically closest to “thoracicus group”, whereas notabilis, snethlageae and devius apparently closer to lafresnayanus of dry interior (which region also includes hellmayri and major). Proposed races omissus (E Brazil) and guttistriatus (S Goiás, Brazil) both included in major. Form successor (described as race of C. procurvoides) is a junior synonym of notabilis (1). Twelve subspecies recognized.Subspecies
Campylorhamphus trochilirostris brevipennis Scientific name definitions
Distribution
Campylorhamphus trochilirostris brevipennis Griscom, 1932
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- brevipennis
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris venezuelensis Scientific name definitions
Distribution
Campylorhamphus trochilirostris venezuelensis (Chapman, 1889)
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- venezuelae / venezuelana / venezuelanus / venezuelense / venezuelensis
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris thoracicus Scientific name definitions
Distribution
Campylorhamphus trochilirostris thoracicus (Sclater, 1860)
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- thoracica / thoracicus
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris zarumillanus Scientific name definitions
Distribution
Campylorhamphus trochilirostris zarumillanus Sztolcman, 1926
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- zarumillanus
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris napensis Scientific name definitions
Distribution
Campylorhamphus trochilirostris napensis Chapman, 1925
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- napensis
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris notabilis Scientific name definitions
Distribution
Campylorhamphus trochilirostris notabilis Zimmer, 1934
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- notabilis
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris snethlageae Scientific name definitions
Distribution
Campylorhamphus trochilirostris snethlageae Zimmer, 1934
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- snethlageae
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris devius Scientific name definitions
Distribution
Campylorhamphus trochilirostris devius Zimmer, 1934
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- devius
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris lafresnayanus Scientific name definitions
Distribution
Campylorhamphus trochilirostris lafresnayanus (d'Orbigny, 1847)
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- lafresnayana / lafresnayanus / lafresnayei / lafresnayi / lafresnayii
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris hellmayri Scientific name definitions
Distribution
Campylorhamphus trochilirostris hellmayri Laubmann, 1930
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- hellmayri
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris major Scientific name definitions
Distribution
Campylorhamphus trochilirostris major Ridgway, 1911
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
- major
The Key to Scientific Names
Legend Overview
Campylorhamphus trochilirostris trochilirostris Scientific name definitions
Distribution
Campylorhamphus trochilirostris trochilirostris (Lichtenstein, 1820)
Definitions
- CAMPYLORHAMPHUS
- trochilirostris
The Key to Scientific Names
Legend Overview
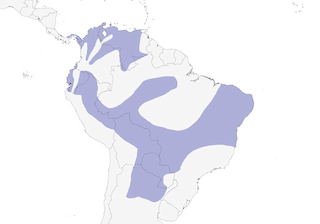
Distribution
Editor's Note: Additional distribution information for this taxon can be found in the 'Subspecies' article above. In the future we will develop a range-wide distribution article.
Habitat
Occurs in a variety of wooded habitats, both in lowlands and adjacent foothills, but usually in relatively open situations. Frequents Chaco and other open woodlands, deciduous and gallery forests, cerrado, caatinga, forest islands in savanna, forest edge, second growth, and various other relatively scrubby habitats; some populations in humid evergreen forest in lowlands or premontane forest and cloud forest in foothills. In Amazonia, primarily seasonally flooded forest (especially várzea) and early-successional riverside cane (Gynerium), being replaced in adjacent terra firme forest by C. procurvoides; also in dense thickets of bamboo, where ecological separation from that species less clear. Mostly tropical lowlands and foothills to c. 1200 m; occasionally ranges into subtropical zone to 2100 m in Andean foothills and coastal cordilleras of N Venezuela.
Movement
Apparently resident and territorial throughout range.
Diet and Foraging
Diet primarily arthropods and especially soft-bodied items such as spiders, but some vertebrate prey apparently taken. Stomach contents included various beetles, ants, flies, small Lepidoptera, cockroaches (Blattodea), millipedes (Diplopoda), parts of spiders, insect larvae, a small scorpion, a large cicada (Cicadidae), and tiny bones. Most prey for which size could be determined were relatively small, less than 20 mm, but some beetle larvae 18–25 mm. Larger items sometimes battered against tree trunks before being swallowed. Generally alone, occasionally in pairs, and often (but not always) with mixed-species flocks; present in 33% of flocks encountered at one site in Venezuelan llanos. Usually seen as it hitches up trunks and branches from upper undergrowth to subcanopy, sometimes higher or lower. At one site in SE Peru, occurred mainly in bamboo thickets, where foraging was concentrated 5·3 m above ground (about a quarter of way up 20-m canopy) and perches used had average diameter 8·3 cm; prey, however, were taken from slightly slimmer substrates averaging 6·3 cm; nearly 75% of foraging attempts were made on bamboo stalks (55·4% live stalks, 18·5% dead), with remaining 26% on other substrates. Forages primarily by probing holes and crevices in bark and bamboo poles, fallen logs, rotting wood, or clumps of moss, bromeliads or other epiphytes; in Peruvian study, over 90% of attacks on prey involved probing, with most of rest gleaning (but occasionally by prying off bits of substrate). Most prey taken from holes in bamboo poles made by, among others, various woodpeckers (Picidae), Amazon bamboo rat (Dactylomys dactylinus) or Peruvian Recurvebill (Syndactyla ucayalae) (46·8%), remaining substrates were bamboo nodes (19·4%) and internodes (8·1%), and non-bamboo stems (25·8%).
Sounds and Vocal Behavior
Song highly variable, both geographically and motivationally. Variation best documented in Venezuela, where two different songs may be given by same bird, one a rapid descending whinny of 10–25 somewhat musical notes lasting 2–2·5 seconds before ending with several slower notes, e.g. “we’he’he’he’he’he’he’e’e’e’e’e” or “tuwee-tuwee-toowa-tew-tew-tew-tew-tew-tew-tew-tew”, and the other (apparently given by agitated birds) a rapid staccato trill (30–35 notes, 2–2·5 seconds) sharply ascending, slowing slightly and sometimes doubled, “dedede’e’e’e’e’e” or “stri’i’i’i’i’i’I’I’I’K!” (similar in quality and pattern to song of Xiphorhynchus obsoletus). In W Ecuador and NW Peru song is instead a descending and gradually slowing series of fewer whistled notes, “tuwee-tuwee-toowa-tew-tew”. In S of range a similar series of longer, clearer whistles that descend only slightly: in Brazilian Pantanal 13–15 notes are given in 2·5–3 seconds and described as “eep, eeep, eeep, eeep, eeep, pee, pee, pee, pee, pee, pee, pew”, in N Bolivia and N Argentina songs are similar in quality but possibly not in timing. When agitated, sometimes gives a descending series of loud, musical “chip” notes; when alarmed, often emits an explosive “chi-dik” that may be repeated.
Breeding
Breeding in May–Jul in N Venezuela; nest with eggs in late Oct in Argentina (Santa Fe); immature in late Jan in Paraguayan Chaco; birds in breeding condition in Feb–Oct in Panama and N Colombia, and in Sept to mid-Nov in S Brazil (Mato Grosso do Sul, where moulting flight-feathers in May), N Bolivia, Paraguayan Chaco and N Argentina. Nest in cavity in tree or stump; one was located in hollow stump 1 m tall; another was 3·5 m up in a hollow Gleditsia tree, with lining of leaves and other herbaceous material from same tree. Clutch 2 smooth, white eggs, occasionally 1 or 3, size apparently varying, from 24 × 21 mm to 30 × 21 mm. A male found with a brood patch suggests extended pair-bond with parental care shared by both sexes.
Conservation Status
Not globally threatened. Uncommon to fairly common but locally distributed throughout most of range; quite scarce within limited region of Central America where it occurs, and scarce at upper end of elevational range. Densities at one site in SE Peru estimated at 17·6 territories/100 ha within stands of bamboo, but 8·5 territories/100 ha on site as a whole; another study in same region obtained comparable estimate of 9 territories/100 ha in early-successional growth along rivers. By contrast, much lower estimates for mature floodplain-forest, e.g. 0·5–1·4 pairs/100 ha, probably reflect patchy distribution of bamboo and presence of species only on small fraction of plot. At least some populations believed to be highly sensitive to human disturbance. Considered an indicator species for tropical lowland humid forest in Chocó lowlands of NW South America, and for bamboo thickets in both N & S Amazonia.
- Year-round
- Migration
- Breeding
- Non-Breeding