Tahiti Reed Warbler Acrocephalus caffer Scientific name definitions
- VU Vulnerable
- Names (18)
- Monotypic
Text last updated August 9, 2017
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | boscarla de Tahití |
| Dutch | Langsnavelkarekiet |
| English | Tahiti Reed Warbler |
| English (United States) | Tahiti Reed Warbler |
| French | Rousserolle à long bec |
| French (France) | Rousserolle à long bec |
| German | Langschnabel-Rohrsänger |
| Japanese | ハシナガヨシキリ |
| Norwegian | tahitisanger |
| Polish | trzciniak długodzioby |
| Russian | Таитянская камышовка |
| Serbian | Trstenjak sa Tahitija |
| Slovak | trsteniarik dýkozobý |
| Spanish | Carricero de Tahití |
| Spanish (Spain) | Carricero de Tahití |
| Swedish | tahitisångare |
| Turkish | Tahiti Kamışçını |
| Ukrainian | Очеретянка довгодзьоба |
Acrocephalus caffer (Sparrman, 1786)
Definitions
- ACROCEPHALUS
- cafer / caffer / caffra
The Key to Scientific Names
Legend Overview
Field Identification
17–20 cm. A large, slender warbler with very long bill , short rounded wings and long tail. Adult has primrose-yellow supercilium, distinct dark eyestripe; crown and upperparts mottled olive-brown, feathers with pale fringes (broadest on upperwing-coverts and tertials); primaries dark brown, rectrices dark brown with yellowish-white tips; throat and underparts are light primrose-yellow; iris dark; maxillae horn-brown, mandible pinkish; legs greenish slate. Worn plumage is slightly darker than fresh plumage. Dark morph has plumage entirely dark brown or sooty black, with slightly paler fringes to wing-coverts and tertials and mainly all-black bare parts, has become more numerous since 1920s (initially very rare, but now considerably more abundant, albeit still heavily outnumbered by the typical, yellow morph). Sexes similar, but male averages larger in most measurements, especially wing and tail length. Juvenile is darker than adult, with dull brown iris, a sulphur-yellow wash to the supercilium, head- and neck-sides, and underparts, especially on breast-sides.
Systematics History
Subspecies
Distribution
Tahiti (Windward Group of Society Is).
Habitat
Riverine woodland, hillside forest, Polynesian bamboo (Schizostachyum glaucifolium) thickets, second growth, groves of purau (Hibiscus tiliaceus) and coconut (Cocos nucifera) plantations; absent from secondary and natural forests without bamboos. At least formerly occurred to 1700 m, but generally at elevations of 50–700 m, and now probably confined to altitudes below 600 m. Often found near small rivers and streams.
Movement
Diet and Foraging
Diet insects, also small lizards, small fish and molluscs, crayfish (Astacus), snails; also seeds, fruit and nectar. Forages alone, mainly in canopy , by gleaning, but also known to occasionally catch prey in flight and to seize items on ground. Lizards are dismembered prior to consumption.
Sounds and Vocal Behavior
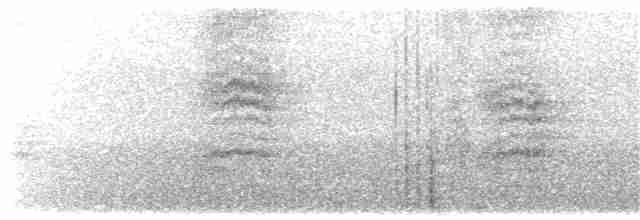
Song a long, comparatively slow-paced (even ‘laboured’) and varied series of musical warbled phrases and “churr” notes, interspersed with louder whistles, the whole somewhat reminiscent (at least to European observers’ ears) like the song of Song Thrush (Turdus philomelos) and rendered “whiteewoo-whiteewoo-wheeo, kaark, gaaark, gaaark, gaaark, whiteewoo-whiteewoo-weeo-weeo”; alarm or other calls include a harsh “chrr”, “gaaark”, “shroo” or “rroo”, often given as prelude to song. Occasionally sings in flight, but usually from a prominent perch.
Breeding
Season believed to be bimodal, peaking between Aug and Dec, and again (to a lesser extent) in Feb–Jun. Appears to be highly territorial year-round. Most nests are constructed in Hibiscus stands or bamboo thickets, and are typically sited up to 15 m above ground. Nest cup-shaped, bulky and larger than those of most Acrocephalus, elaborately constructed from various plant materials, including grass, rootlets and moss, for two nests mean internal diameter 9 cm, height 17 cm, cup depth 12 cm; territorial. No further information.
Conservation Status
ENDANGERED. Previously listed as Vulnerable. Current population estimated at fewer than 1000 individuals, within an overall range of 420 km². Restricted-range species: present in Society Islands EBA. Rare and now largely confined to E & C Tahiti, with concerns already being expressed for the species’ future as early as the first decade of the last century, although it was probably at least locally abundant in the 18th and 19th centuries. Absent from W side of Tahiti and the peninsula. In 1986–1991, estimated to number no more than a few hundred individuals. Became increasingly rare and local throughout 20th century; recorded in six of 14 valleys visited during period 1920–1923, and in 15 of 39 visited during 1986–1991 and again in 2000s, when there were estimated to be no more than 100–300 occupied territories. Ongoing threats are exploitation and loss of bamboo, invasion by the Neotropical weed Miconia, construction of roads, houses and hydroelectric micro-dams, and disturbance created by four-wheel-drive vehicles, all of which have resulted in considerable modification of habitat, with the birds being effectively driven inland; e.g., in Papenoo Valley, in the early 1920s the closest territories to the sea were c. 2 km inland, but were 3 km from the sea by the early 1970s and 6 km inland in 2005, and it has been estimated that several tens of territories were lost in this valley and the Mahaena Valley between the 1970s and 2005. Introduction of several alien bird species, including aggressive Common Myna (Acridotheres tristis) and Red-vented Bulbul (Pycnonotus cafer), likely to have had a notable adverse effect on this warbler; rats, particularly black rat (Rattus rattus), may also be a contributory factor in its rarity.

- Year-round
- Migration
- Breeding
- Non-Breeding