White-naped Honeyeater Melithreptus lunatus Scientific name definitions
- LC Least Concern
- Names (17)
- Monotypic
Text last updated June 7, 2016
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | menjamel de clatell blanc oriental |
| Dutch | Diadeemhoningeter |
| English | White-naped Honeyeater |
| English (United States) | White-naped Honeyeater |
| French | Méliphage à lunule |
| French (France) | Méliphage à lunule |
| German | Mondstreif-Honigfresser |
| Japanese | ハチマキミツスイ |
| Norwegian | hvitnakkehonningeter |
| Polish | miodopoik czerwonobrewy |
| Russian | Краснобровая медвянка |
| Slovak | medárik eukalyptový |
| Spanish | Mielero Nuquiblanco |
| Spanish (Spain) | Mielero nuquiblanco |
| Swedish | vitnackad honungsfågel |
| Turkish | Hilal Enseli Balkuşu |
| Ukrainian | Медопійник червонобровий |
Melithreptus lunatus (Vieillot, 1802)
Definitions
- MELITHREPTUS
- lunatus
The Key to Scientific Names
Legend Overview
Field Identification
10·5–15·5 cm; male 11·5–25 g and female 11·5–15 g. Adult has black cap covering top and sides of head and hindneck, arc of bare bright orange-red skin above eye , short white crescent-shaped band across nape; upperparts and uppertail olive-green, tail with brown sides edged olive; upperwing mostly olive-brown, olive-green tips to coverts and olive-green outer edges to remiges (olive panel on folded wing); chin and throat white, black mark on upper chin; underparts white, dark grey-brown wash on sides of breast and flanks, undertail light brown, underwing white with brown trailing edge and tip; iris brown; bill and gape black; legs brown to black, often tinged orange, especially on feet. Sexes alike in plumage, male larger than female. Juvenile as adult but much duller overall, top of head and hindneck brown, contrastingly darker black-brown mask, light brown to pale orange arc of bare skin over eye, narrower and diffuse off-white to yellowish-buff napeband, upperparts mostly brown (except olive-green uppertail-coverts), outer greater coverts, primary-coverts and alula initially with narrow olive-buff fringes at tips, underparts off-white, bill dull orange with grey-black distal half of maxilla (area of black increasing with age), gape swollen and orange.
Systematics History
Subspecies
Distribution
E & SE Australia from N Queensland (wet tropics) S, extending W to inland slopes of Great Divide and adjacent inland plains, to Victoria and to SE South Australia (including Kangaroo I).
Habitat
Mainly open eucalypt forests and woodlands, especially dry sclerophyll forest or woodland, with patchy or moderately well-developed understorey of shrubs such as Acacia, Banksia, Casuarina or heath; also tall wet sclerophyll forest, often with well-developed understorey of Acacia, and occasionally dense subcanopy of rainforest. Sometimes in riparian woodland of casuarinas; open or coastal Banksia shrubland; heathland with low scattered emergent eucalypts; or alpine herbfields. Found in urban gardens and streets in some areas. Occasionally in plantations of exotic pines.
Movement
Partly migratory and partly sedentary. Over much of range resident, with local movements (often described as locally nomadic), although these populations often subject to some regular seasonal influx and exodus movements, and other areas visited irregularly. Migration apparently confined to populations on and E of Great Divide, from S Queensland or N New South Wales S to Victoria (recorded throughout year in most of this range, and such populations assumed to be at least partly resident), consists of exodus from high altitudes and movement along Great Divide and adjacent slopes and scarps, although sometimes recorded above snowline in Snowy Mts, New South Wales, in winter; many birds return to same area year after year. Migrates in monospecific flocks or in flocks with Caligavis chrysops or, occasionally, Anthochaera carunculata; seen on passage in flocks of up to 100 individuals, and thousands may pass over a given point during one day (e.g. hundreds of thousands of honeyeaters, mainly of present species, moving N along coast at Mallacoota, in E Victoria); flocks usually smaller and more dispersed on S passage. On migration, follows valleys and timbered ridges, or lines of trees.
Diet and Foraging
Arthropods (mainly insects, some spiders) and nectar (including of Eucalyptus, Banksia, Grevillea); occasionally manna, honeydew and lerp. In South Australia, estimated ratio of nectar to insects (latter probably including lerp and honeydew) in diet 24:76. Forages at all heights, mainly in canopy, mostly in trees (particularly Eucalyptus), less often in shrubs (including Banksia, Grevillea, Callistemon, Astroloma) or mistletoe (e.g. Lysiana, Amyema); searches mainly in foliage, particularly outer foliage, on twigs and flowers in crowns of tall eucalypts, less often on branches or trunks; occasionally on ground. Mean foraging height at site in E Australia 9·6 m (with 79% of observations above 4 m). At five sites in E Australia, more than 70% of foraging observations among foliage. At site in E Australia, two phases of foraging activity, first in early morning, characterized by much nectar-feeding and little foraging for insects, and second during rest of day, with slight increase in rates of insect-eating and corresponding reduction in nectar consumption. Insects taken by gleaning from foliage or probing bark, and occasionally by sallying; probes flowers for nectar with rapid circular motion. Often acrobatic , sometimes standing erect or hanging upside-down. Forages singly, in pairs, or in small loose flocks of twelve or more individuals, but can gather in large numbers in areas with abundant flowering plants; also in flocks of hundreds or even thousands on migration. Often forages with other meliphagids, including M. brevirostris and Caligavis chrysops; can be aggressive to other foliage-gleaners.
Sounds and Vocal Behavior
Sometimes noisy, and during breeding season continual noisy aerial chases. Most common call a half-whistled, half-hissed, churring “sherp-sherp-sherp…” or “tserp-tserp-tserp…”, given frequently throughout day. Other calls include short single note with upward inflection, given often during foraging or in flight; short, high-pitched, rather plaintive insect-like “chip”, “tsip”, “tsit” or “twit”, uttered continually as contact during foraging and while in flight during migration; and “joe-joe-joe”, to advertise territory. Alarm call a tense quiet “pew-pew-pew…” or repeated staccato notes.
Breeding
Recorded in all months, but season mainly late winter to early summer, Aug–Jan (eggs Jul–Jan); usually double-brooded. Regularly breeds co-operatively and semi-colonially, but also nests solitarily. Nest probably built by female alone, sometimes accompanied by other birds, open and cup-shaped or purse-shaped, sometimes deep, usually woven from grass, bark or spider web, sometimes with feathers, moss, plant down, lichen or leaves matted into structure, lined with plant down, fine grass, rootlets, small leaves, feathers, hair, fur, bark, wool or moss, sometimes unlined, external diameter 5·1–6·4 cm, depth 3·5–7·6 cm, internal diameter 3·8–4·4 cm, depth 3·8–4·4 cm; usually suspended by rim among outer foliage high above ground, sometimes in low branches, usually in tree or sapling (especially Eucalyptus) but sometimes in pendulous foliage of mistletoe, 0·2–40 m (mean 9·5 m) above ground. Clutch 2–3 eggs, mean 2·67; incubation probably by female only, period poorly known but probably 10–14 days; chicks fed by both sexes, and by auxiliaries if present, nestling period c. 14–16·5 days. Nests parasitized by Pallid (Heteroscenes pallidus) and Fan-tailed Cuckoos (Cacomantis flabelliformis). From 24 eggs in ten nests (perhaps mainly this species but possibly also including M. chloropsis), 0·7 young fledged per nest; of 45 nests at which outcome known, 30 (67%) fledged at least one young. Maximum longevity at least 12 years.
Conservation Status
Not globally threatened (Least Concern). Common. No estimates of total population but recorded densities of up to c. 5 birds/ha and, exceptionally, 12·1 birds/ha. In counts along a 200-km transect through C Victoria, mean densities 0·57 birds/ha in summer, 0·57 in autumn, 0·47 in winter, and 0·38 in spring, with maximum recorded density 3·9 birds/ha; at another site in C Victoria maximum 12·1 birds/ha, yet few birds present for ten months of the year in which this density recorded. After experimental removal of Manorina melanophrys from sites near Melbourne (S Victoria), present species moved into vacated sites to forage on psyllids. Local, habitat-specific declines reported in mountains of South Australia. Reports of this species from Kent Group, in Bass Strait, assumed to be referable to misidentified M. validirostris or M. affinis.
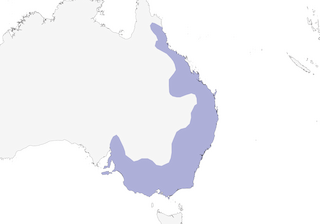
- Year-round
- Migration
- Breeding
- Non-Breeding