Dusky Thrush Turdus eunomus Scientific name definitions
- LC Least Concern
- Names (39)
- Monotypic
Text last updated December 4, 2014
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Arabic | سمنة معتمة |
| Bulgarian | Кафяв дрозд |
| Catalan | tord alabrú |
| Chinese | 斑點鶇 |
| Chinese (Hong Kong SAR China) | 斑鶇 |
| Chinese (SIM) | 斑鸫 |
| Croatian | šareni drozd |
| Czech | drozd rezavokřídlý |
| Danish | Brundrossel |
| Dutch | Bruine Lijster |
| English | Dusky Thrush |
| English (United States) | Dusky Thrush |
| Faroese | Mortrøstur |
| Finnish | ruostesiipirastas |
| French | Grive à ailes rousses |
| French (France) | Grive à ailes rousses |
| German | Rostflügeldrossel |
| Hebrew | קיכלי חום-כנף |
| Hungarian | Rőtszárnyú rigó |
| Icelandic | Brúnþröstur |
| Italian | Cesena fosca |
| Japanese | ツグミ |
| Korean | 개똥지빠귀 |
| Lithuanian | Dūminis strazdas |
| Mongolian | Хүрэн хөөндэй |
| Norwegian | svartflekktrost |
| Polish | drozd rdzawoskrzydły |
| Portuguese (Portugal) | Tordo-sombrio |
| Romanian | Sturz cu aripi ruginii |
| Russian | Бурый дрозд |
| Serbian | Sivi sibirski drozd |
| Slovak | drozd sibírsky |
| Slovenian | Rdečeperuti drozg |
| Spanish | Zorzal Eunomo |
| Spanish (Spain) | Zorzal eunomo |
| Swedish | bruntrast |
| Thai | นกเดินดงอกลายดำ |
| Turkish | Karaca Ardıç |
| Ukrainian | Дрізд темний |
Turdus eunomus Temminck, 1831
Definitions
- TURDUS
- turdus
- eunomus
The Key to Scientific Names
Legend Overview
Field Identification
23–25 cm; 55–106 g. Male is boldly blackish and white on head, with black of crown and ear-coverts meeting on nape, whitish supercilium , subocular line, broad submoustachial and throat , narrow black-spotted malar streak; upperparts blackish with narrow rufous scalloping, rufous wings ; blackish on breast and flanks , increasingly broad whitish scalloping on latter towards vent, off-white belly to vent; bill dark, yellow lower mandible; legs dark brown. Intermediates with T. naumanni occur in extensive contact zone between breeding populations, many resembling present species but with mantle, back and scapulars heavily washed deep copper-brown, median and greater wing-coverts golden-brown. Female is like male, but brown above and on head, no rufous in wings , less white on throat. Juvenile is as adult, but extensively spotted buff above and blackish below, without adult’s black breastband; first-winter like female, but with blackish feather centres on much of upperparts.
Systematics History
Subspecies
Hybridization
Hybrid Records and Media Contributed to eBird
-
Dusky x Naumann's Thrush (hybrid) Turdus eunomus x naumanni
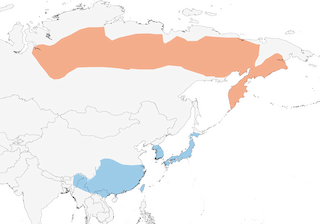
Distribution
NC & NE Siberia from lower R Yenisey E to W Chukotka and Kamchatka; S limit uncertain but includes middle Angara basin, middle and upper R Podkamennaya Tunguska and upper R Lena, and possibly S to L Baikal, and extending E to at least upper Vitim. Non-breeding from NE India E to N Vietnam (E Tonkin), SE & E China (mainly S of Yangtze basin), Taiwan, Korea and Japan.
Habitat
Breeds in lowland tundra edges, scrub, dense riverine stands of willow (Salix) and poplar (Populus) and wooded steppe, sometimes open coniferous woodland, and also reaching more open areas; habitat similar to that of T. naumanni, but generally extending into more montane terrain. Winters in hillside scrub, open grassy areas, sparse woodland, cultivated farmland, orchards and grassland with scattered trees, suburban parks and gardens, from foothills to 3000 m.
Movement
Migratory. Winters from S Japan and Taiwan S & W through S China, NW Thailand, N Myanmar and E Himalayan foothills; small numbers winter in E Siberia. Quits Kamchatka Peninsula first half Sept, with passage in Ussuriland mid-Sept to mid-Oct and around Krasnoyarsk (SC Siberia) early Oct. In NE China, main autumn passage in Heilongjiang Sept and first three weeks Oct, but in Beidaihe mid-Oct to mid-Nov (c. 2 weeks later than that of T. naumanni); bypasses Korean Peninsula in autumn, but occurs in S Korea in Apr. Large flocks arrive in Japan late Sept, with peak from mid-Oct to Nov, spreading down to lower elevations to reach Kanto Plain and other lowland areas farther S for winter; spring passage covers mid-Mar to early May. In Hong Kong a scarce winter visitor, with 89% of records Jan–Mar; pattern rather irregular and irruptive. Winters in low numbers in Himalayas (rare and irregular in W, scarce in E) from N Pakistan E to NE India. Spring passage extends from mid-Mar in Russian Far East, where generally a few days later than T. naumanni, reportedly not appearing around L Khanka (SE Russia) until last third of Apr and continuing through into mid-May; arrives on breeding grounds middle to end May. Vagrants recorded Europe, Middle East, Philippines (1) and NW North America.
Diet and Foraging
Stomachs from China, in period Apr–Nov, held beetles (Coleoptera), locusts (Locustidae), insect larvae and plant seeds; in Nov–Feb, fruits and seeds, including juniper (Juniperus) and ash (presumably Fraxinus), but also beetles, ants (Hymenoptera), earthworms and spiders. On passage in Russia, takes chiefly grapes and buckthorn berries, also rowanberries. Forages largely on ground .
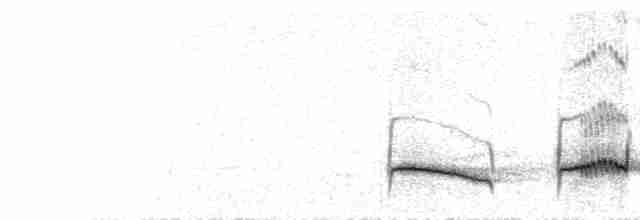
Sounds and Vocal Behavior
Song , by male from high in tree, a series of short, loud, musical phrases of 3–5 notes which include rich melancholy flutings, downslurred whistles and dry trills, also sometimes much more sustained and rich, with quality reminiscent of song of Luscinia megarhynchos, T. merula or T. philomelos, “veet tyulir-tyulir fru-fru fir-fee veet-veet tyulir-tyulir che-che-che-che veet-veet-veet fru-fru- pryupee-pryupee…”; apparently more powerful and varied than that of T. naumanni (but latter inadequately known, and comparison therefore provisional). Calls include subdued staccato “chuck” notes in mild alarm or as warning, strident rhythmic “chek-chek-chek-chek” e.g. when going to roost, conversational “kveveg” or “wäwä” for contact (often in flight) when often becomes variable musical chattering, also dry shrill rasping “shrrrt!” or “spirr” on flushing.
Breeding
May/Jun–Aug. Nest a crude cup of various grasses, twigs and moss, mixed with mud and lined with fine grass, placed up to 5 m above ground, but often below 1 m, in small isolated tree. Eggs 4–6, greenish-blue with reddish-brown streaks. No other information.
Conservation Status
Not globally threatened. Common. In winter rare in N Korea, commoner in S Korea (mainly spring). Common to abundant on S Kurils, Hokkaido , Honshu, Sado, Shikoku, Kyushu and the smaller islands of Japan; has recovered from the persecution that lasted until 1947, when up to 5 million taken annually for the grilled-bird trade, which considerably suppressed wintering numbers. In China, the most abundant thrush in winter in Fujian and common in Shanghai area. Scarce visitor in Myanmar, NW Thailand, N Vietnam. Hunted for food in rural China.
- Year-round
- Migration
- Breeding
- Non-Breeding